Chromium (Cr) is a metal with a unique relationship with the environment. While trivalent chromium (Cr(III)) is a necessary nutrient, hexavalent chromium (Cr(VI)) is toxic to humans and aquatic life, posing major environmental and ecological risks.
Recent studies of various ground and drinking water sources have revealed dangerous Cr(VI) levels.
This alarming tendency has piqued the interest of national and international health authorities, including the United States Environmental Protection Agency (EPA) and the US Food and Drug Administration (FDA), which want to know how widespread the problem is.
In 2001, the California Department of Public Health added Cr(VI) to the uncontrolled chemicals requiring monitoring.
According to recent data, 3,107 of 6,565 public wells in Los Angeles, San Bernardino, and Fresno counties had Cr(VI) concentrations above 1 μg/L. The Public Health Goal of 0.02 μg/L was published in July 2011.
The element's nature and sample matrices' diversity make chromium species analysis problematic. Because chromium exists in two oxidation states, it is essential to distinguish between the nutritional Cr(III) and the toxic Cr(VI) in samples.
A (HPLC-ICP-MS) method using the Hamilton PRP-X100 was developed to assess the relative abundance of Cr(III) and Cr(VI) in various sample matrices.
Trivalent chromium (Cr(III)) is stabilized as a chelation complex by incubating the sample in 0.2 mM EDTA at 70 °C. The Cr(III)-EDTA complex is excellent for binding with an anion exchange resin.
The resolution of the two species then becomes a simple isocratic separation. Although UV and conductivity can identify chromium species, inductively coupled plasma-mass spectrometry (ICP-MS) is the preferred approach for trace measurement.
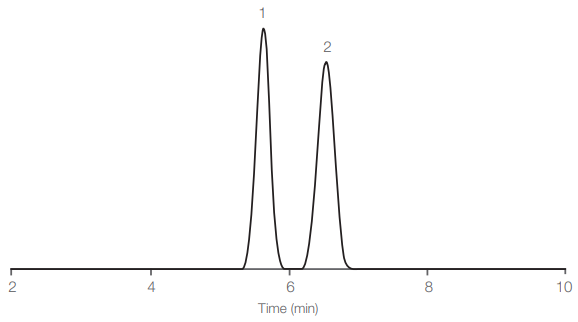
1) Cr(VI) and 2) Cr(III) EDTA separation on Hamilton PRP-X100. Image Credit: Hamilton Company
Experimental Conditions. Source: Hamilton Company
| . |
. |
| Column |
Hamilton PRP-X100, 5 µm, 4.6 x 250 mm |
| Part Number |
79181 |
| Flow Rate |
1.0 mL/min |
| Mobile phase |
2 mM (NH4)2 CO3 for 0 – 3 min
40 mM (NH4)2 CO3 for 3 – 14 min
2 mM (NH4)2 CO3 for 13 – 17 min. |
| Injection Volume |
50 µL, 100 µg/L of each standard |
| Detection |
ICP-MS |
PRP-X100 HPLC Column Ordering Information. Source: Hamilton Company
| Column Type |
Hardware Size (mm) |
Particle Size |
| 5 µm |
10 µm |
12 – 20 µm |
| PRP-X100 |
2.1 x 150 PEEK |
79852 |
|
|
| PRP-X100 |
4.6 x 150 PEEK |
79174 |
79354 |
|
| PRP-X100 |
4.6 x 250 PEEK |
79181 |
79455 |
|
| PRP-X100 |
Bulk Resin (1 Gram) |
79584 |
79585 |
79586 |
PRP-X100 HPLC Guard Column Ordering Information. Source: Hamilton Company
| Part Number |
Description |
| 79383 |
Analytical Guard Column Starter Kit (1 holder, 2 cartridges), PEEK |
| 79385 |
Analytical Guard Column Replacement Cartridges (5/pk), PEEK |

This information has been sourced, reviewed and adapted from materials provided by Hamilton Company.
For more information on this source, please visit Hamilton Company.