Catechins present in tea (green, white, and black) are well-known for their antioxidant qualities. Catechins have been demonstrated to have antibacterial efficacy against staphylococcus, E. Coli, and H. pylori1 while also inhibiting oxidative degradation.
Catechins' properties have made them useful in a variety of applications, including commercial teas, food preservation,2 free radical scavenging,3, and disinfection.4 However, certain quantification procedures require HPLC run lengths of more than 20 minutes.
To improve HPLC resolution after catechin extraction from a commercial green tea bag, a 10 mM (pH 2.5) sodium phosphate buffer for the aqueous mobile phase and acetonitrile as an organic eluent was used.
The optimal separation temperature was found to be 34 °C for the best resolution and peak form. All eight compounds were isolated within 12 minutes. Under these conditions, the third caffeine peak is extremely sensitive to temperature variations.
Higher temperatures resulted in higher retention; however, chilling the column to less than 20 °C reduced retention to less than 4 minutes while keeping the remaining peak retentions roughly constant.
Reproducibility tests are provided for the analysis from the first to the 500th injection, illustrating the durability of all Hamilton Company HPLC columns.
Source: Hamilton Company
| Column Information |
| Packing Material |
PRP–C18 (5 μm) |
| Dimensions |
150 x 4.6 mm |
| P/N |
79676 |
| Chromatographic Conditions |
| Gradient |
0.0–15.00 min. 10–25 % B |
| Temperature |
34 °C |
| Injection Volume |
5 µL |
| Detection |
UV at 214 nm |
| Eluent A |
10 mM NaH2 PO4 (pH= 2.5) |
| Eluent B |
Acetonitrile |
| Flow Rate |
2.0 mL/min |
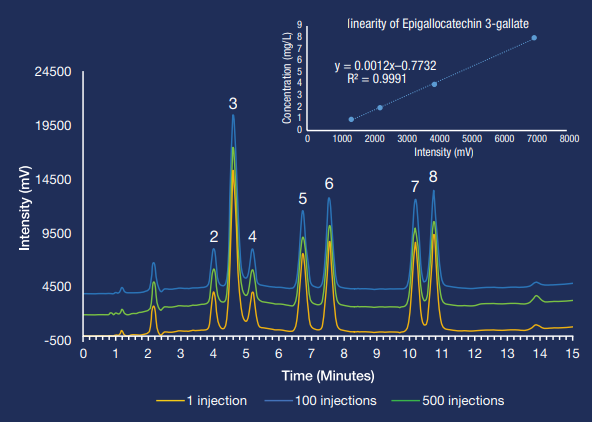
Compounds: 1: Gallocatechin 2: Catechin 3: Caffeine 4: Epicatechin 5: Epigallocatechin 3-gallate 6: Gallocatechin 3-gallate 7: Catechin 3-gallate 8: Epicatechin 3-gallate. Image Credit: Hamilton Company
References
- Jeon J., Kim J. H., Lee C. K. Annals of Dermatology. 2014, 26(5), 564.
- He, Y. H., Shahidi, F. J. Ag. Food Chem. 1997, 45(11), 4262.
- Gupta D. A., Bhaskar D. J., Gupta R. K. Bio. Sci. Pharm. Res. 2014, 2, 8.
- Reygaert, W. C. Bio. Med. Res. Int., 2018 9105261.

This information has been sourced, reviewed and adapted from materials provided by Hamilton Company.
For more information on this source, please visit Hamilton Company.