The identification of polymeric hardening agents has resulted in unexpected consequences for humanity. Compared to other modalities, plastic containers have low initial costs and may be formed into any requested shape.
Amphiphilic compounds are added to help the container retain its shape and prevent it from corrosion caused by acidic components. However, because chemicals are added to the polymer to control its hardness, pliability, lifetime, and other plasticity metrics, leaching is risky under certain situations.
These conditions include higher solvent temperatures, lipophilic and amphiphilic materials, and prolonged exposure to UV radiation.
Bisphenolic hardeners have been linked to endocrine disruption in animal and human reproduction cycles.
Bisphenol A (BPA), which includes a phenol bonded to another phenol by a dimethyl methylene bridge similar to phenolic alcohols, has received the greatest attention from the FDA and has been shown to cause endocrine disruption. The FDA recommends that adults limit their oral intake to 5 mg/kg body weight per day.1
BPA ingestion has been related to the disruption of male and female reproduction due to its weak estrogenic characteristics.2 Similarly, several bisphenolic plasticizers have shown some early signs of being estrogen mimics.3
Over the last decade, much research has been conducted to investigate the effects of BPA on human health and the environment. However, research into alternate bisphenols utilized in polycarbonate (PC) bottles has not proven exhaustive.
Due to unforeseen consequences, industrial plastics manufacturers who use BPA were forced to adapt. However, the bisphenols substituted for BPA, now common in the plastic industry, are equally harmful.
Initial research indicates that bisphenol variations have equal health and environmental impacts, but they are not widely known to the public.4
Some bisphenolic derivatives are S, F, M, or Z (BPS, BPF, BPM, BPZ). When subjected to extreme temperatures, UV light, and oil-based environments, bisphenolic compounds leach in the same way that BPA does.5
In one investigation, bisphenol derivatives were subjected to standard leaching tests typically done with BPA. BPZ values in those investigations, either from a hot water leachate solution or a 50 % ethanol solution, ranged between 1.1 and 4.6 ng/L, which is comparable to BPA.6
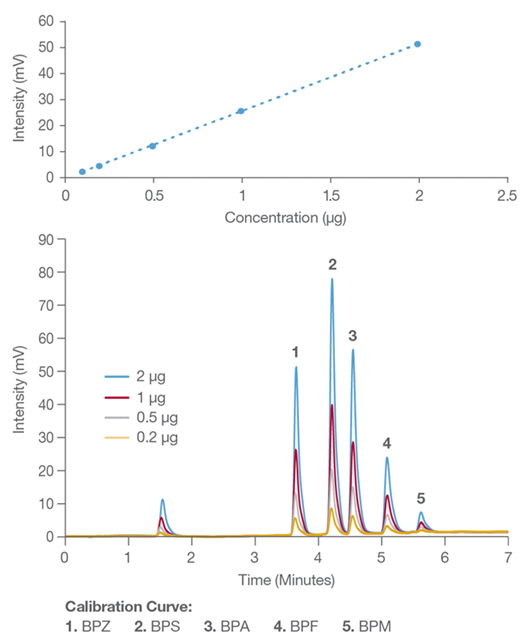
Figure 1. HPLC chromatogram of the five bisphenol standards. Image Credit: Hamilton Company
This article aims to determine if BPA-free bottles are indeed BPA-free.
- How much BPA or similar compounds are in the sample?
- Is it possible to remove BPA or its compounds from a bottle sample?
- How does purging the leached material affect the bottle's structural integrity?
Considering these questions, a baseline set of tests was designed to be carried out on a Shimadzu 20A HPLC apparatus with UV detection. The study used a Hamilton Company 50 x 7.8 mm column with a 5 μm PRP-1 particle and guard column for quick separation and identification of bisphenol compounds (Bisphenol A, Z, F, M, and S).
The eluents were acetonitrile and distilled water. Samples were generated by adding 200 mL of water at 25, 35, 50, or 70 °C or a varied concentration of ethanol (5, 20, or 40) to a 1 L BPA-free plastic water bottle. The extraction solution was added, vigorously mixed for two minutes, and then left to rest for 30 minutes.
The material was then put into a glass beaker, and a 20 mL aliquot was extracted and concentrated using an SPE cartridge (eluted with 1:1 acetonitrile: water).
Method validation was accomplished using the same process described above, with a 1 mg/mL spike solution dissolved in a 200 mL extraction solution supplied to the water bottle at 25 °C.
Initial spike recoveries were computed as +/-95-105 % of the initial spike concentrations.
Column Information. Source: Hamilton Company
| |
|
| Packing Material |
PRP-1, 5 μm |
| Dimensions |
50 x 7.8 mm |
| P/N |
79642 |
Chromatographic conditions. Source: Hamilton Company
| |
|
| Gradient |
0.0–1.0 min. 16 %
B 1.0–5.0 min. 16–80 %
B 5.0–7.0 min. 80 %
B 7.0–9.0 min. 100 % B |
| Temperature |
35 °C |
| Injection Volume |
10 μL |
| Detection |
UV at 230 nm |
| Eluent A |
Water |
| Eluent B |
Acetonitrile |
| Flow Rate |
2.0 mL/min. |
The experimental results of the initial bisphenol leaching test on new BPA-free PC water bottles were studied using HPLC detection.
Extractions with water at 25, 35, 50, and 70 °C are based on possible seasonal temperature changes encountered inside an automobile. Bisphenolic compounds did not leach after being extracted at 25 °C in seven fresh PC plastic bottles.
The extraction data (Table 1) show a linear relationship between extraction temperature and bisphenol concentrations. This connection represents the degree of lability of the bisphenolic analytes in the polymeric web containing the plasticizer.
Confirmation of BPA-free bottles containing BPA derivatives raised the question: Can hot water rinses reduce BPA derivative leaching? To test this theory, the bottles were periodically exposed to 70 °C water at 30-minute intervals until the extracted bisphenol concentration fell below the detection limit.
Table 1. Water Temperature Versus Leachate Concentration. Source: Hamilton Company
| Temperature (°C) |
Bisphenol Concentration (μg) |
| 25 |
<0.001 |
| 35 |
0.027 |
| 50 |
0.045 |
| 70 |
0.064 |
The results in Table 2 show that after four consecutive exposures to 70 °C water for 30 minutes, the bisphenol concentration is below the detection limit.
This finding suggests that regularly exposing a reusable bottle to near-boiling water may remove leachable bisphenol. A crude investigation of the bottle revealed no structural damage to the PC bottle.
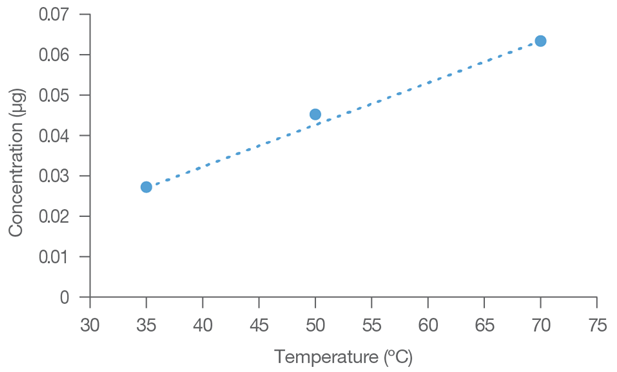
Figure 2. Trend line for water temperature vs. concentration of extracted bisphenol. Image Credit: Hamilton Company
Because of the global prevalence of alcoholic beverages, research into leachates with ethanol is of particular relevance. Furthermore, when drinking away from home, many people fill their water bottles with various types of alcoholic beverages.
With the constant risk of shattered glass, most outdoor facilities prohibit glass containers, pushing plastic beverage holders.
The three most frequent ethanol percentages on the market were analyzed: 5, 20, and 40 %, found in beer, wine, and distilled alcohols such as whiskey or vodka. The extraction temperature of 25 °C was chosen because bisphenol is more solubilized in ethanol.
Table 2 shows a linear relationship between extracted bisphenol content and the rising proportion of ethanol in new PC bottles. These results are unsurprising given that bisphenolic derivatives are more soluble in amphiphilic solvents than water.
A comparison of the two trends, hot water versus ethanol, revealed that the two data sets produce comparable linear relationships but with a higher bisphenol content extracted using ethanol than hot water. Table 3 shows that repeated exposure to 40 % ethanol gradually decreases bisphenol leachate per interval.
In this study, five washes of 40 % ethanol removed bisphenolic residues to an undetectable level while retaining the bottle's structural integrity (Figure 3).
Table 2. Bisphenol Leachate Concentration Versus Percent of Ethanol in the Extraction Solution in a New Bottle. Source: Hamilton Company
| Ethanol Percent |
Bisphenol Concentration (μg) |
| 5 |
0.874 |
| 20 |
1.206 |
| 40 |
1.527 |
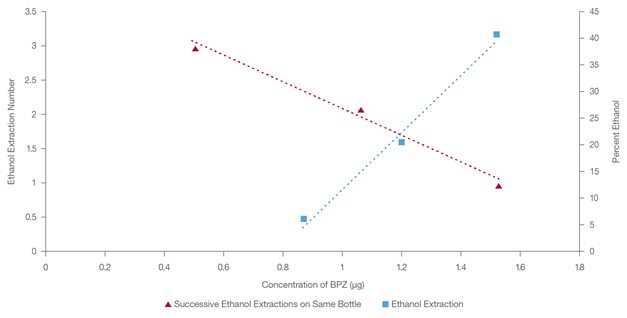
Figure 3. Concentration of bisphenol on x-axis versus the number of extraction on the same bottle (y1-axis) versus percentage of ethanol used in a new bottle extraction (y2-axis). Image Credit: Hamilton Company
Finally, a PC bottle leached by successive 70 °C water was extracted using a 40 % ethanol solution. The ethanol extraction yielded an additional 1.27 ± 0.05 μg of bisphenolic compounds from the purged bottle.
Attempting to extract more bisphenol with hot water following depletion with ethanol resulted in (<0.01 ± 0.05 μg) no more bisphenol in the 30-minute exposure. This study shows that liquid chromatography can quickly and easily detect bisphenolic substances.
According to the mini study, new water bottles labeled BPA-free contain bisphenol derivatives that can leach when exposed to alcohol or water at temperatures over ambient. Water must be kept at or below 25 °C to eliminate the risk of leaching harmful bisphenol.
In addition, leachable bisphenols were removed from new polycarbonate bottles by washing them with 40 % ethanol five times. The results were satisfactory, with values below the study's detection limit of 0.05 μg while maintaining the container's structural integrity.
References
- https://wayback.archive it.org/7993/20180126150108/https://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-0038b1_01_02_FDA%20BPA%20Draft%20Assessment.pdf.
- Lacroix MZ, Puel S, Collet SH, Corbel T, Picard-Hagen N, Toutain PL, Viguié C, Gayrard V. Talanta. 2011, 85(4):2053–9.
- Andra SS, Austin C, Yang J, Patel D, Arora M. Sci Total Environ. 2016, 572:770–781.
- Wang G, Qi P, Xue X, Wu F, Deng N. P. Chemosphere. 2007, 67(4):762–9.
- Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, Huttner K, Hu H. Envir. Health Perspect. 2005,113(9):1222–5.
- Kovačič A, Gys C, Rafael Gulin M, Kosjek T, Heath D, Covaci A, Heath E. Food Chem. 2020, 331(30), 127326.

This information has been sourced, reviewed and adapted from materials provided by Hamilton Company.
For more information on this source, please visit Hamilton Company.