Updated 23/11/22 by Reginald Davey
Updated 12/02/2020 by Jo Finchen-Parsons
This article will look at the phenomenon of metallic corrosion in mild steel, a key engineering material used in construction and equipment manufacturing. It will also provide a brief overview of mild steel, its properties, and applications.

Image Credit: PHATRAWUTH EAWCHAREON/Shutterstock.com
Mild Steel - An Overview
Mild steel is a material that is utilized in several industrial applications. It is a cost-effective steel with a carbon content of 0.16-0.29% and has a relatively high melting point of 1450-1520oC. Due to its low carbon content, it has a higher melting point than other high-carbon content steels.
The high melting point of mild steel gives it enhanced ductility when it is heated, which makes it suitable for processes such as cutting, welding, forging, and drilling. Easy to manufacture, it is not however suitable for through hardening. By adding chemically reactive carbon sources when heating, mild steel can be case hardened.
There are several different commercial grades of mild steel, but they all possess the above-mentioned carbon content. Three stages are used in the production of mild steel: primary steelmaking in basic oxygen furnaces and electric arc furnaces, secondary steelmaking, where carbon content is reduced and alloying elements are added as a final casting and forming step.
Secondary forming is then used to improve the mechanical properties of mild steel to meet specific application requirements. Secondary forming methods include machining, cold rolling, coating, tempering, and surface treatments.
Physical Properties
Mild steel has many beneficial physical properties. These include it high tensile strength, magnetic properties, good malleability, ductility, weldability, and high impact strength. As well as its favorable physical properties, mild steel can be easily recycled.
Uses of Mild Steel
Mild steel is used in several applications and is supplied in forms such as pipes, sheets and T sections. It is used in the construction industry for structural elements as it can withstand changing loads and meets strict requirements for materials which can withstand environmental factors such as wind and earthquakes. It is also used in pipelines, poles, machinery and automobile manufacturing, fencing, cutlery, and cookware.
Does Mild Steel Rust? An Overview of Corrosion
Does mild steel rust? Does carbon steel rust? Like all variants of steel and iron-based materials, the answer is yes, but this issue can be overcome.
If mild steel is exposed to an aerated neutral aqueous solution, such as a dilute solution of sodium chloride in water, a corrosive attack will begin at defects in the oxide film on the steel. These defects may be a result of mechanical damage, such as scratches, or natural discontinuities in the film, such as inclusions, grain boundaries, or dislocation networks present on the surface of the steel.
At each defect, the steel is exposed to the electrolyte solution and an anodic reaction occurs, thereby resulting in the formation of iron ions and free electrons. These electrons are then conducted through the oxide film, where they participate in a cathodic reaction at the surface of the film. This reaction requires the presence of dissolved oxygen in the electrolyte solution and ultimately results in the formation of hydroxyl ions.
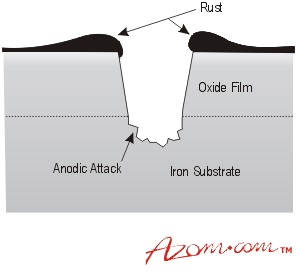
Rusting of mild steel.
The hydroxyl ions react with the ferrous ions produced by the anodic reaction to form ferrous hydroxide, which is then converted into a hydrated oxide commonly referred to as ‘rust'. Gradually, a scab of rust may form over the top of the pit; however, it is too porous to completely block the anodic area. Therefore, the corrosion process can continue, resulting in a deeper attack and widening of the anodic area as the surface oxide film breaks away.
If the pH of the solution in contact with the steel is low, as would be the case if a dilute acid is used, then the surface oxide film will be removed and the cathodic reaction will be different. Hydrogen gas will be liberated as the gradual dissolution of the steel occurs. With oxidizing acids, a number of alternate cathodic reactions may take place.
In all cases of corrosion, the anodic reaction cannot proceed in isolation from the cathodic reaction. Furthermore, if either reaction can be limited or stopped, then less or no corrosion will occur.
How to Improve Mild Steel’s Corrosion Resistance
In its untreated form, this material is highly susceptible to rusting. To improve the corrosion resistance of mild steels, several strategies can be employed. Appropriate surface coatings can be applied to protect this material, with red oxide primers, zinc treatments, and metal paints available commercially for enhancing corrosion resistance and the aesthetic appeal of mild steel.
A process referred to as “pickling” can be used to clean mild steel and extend its life. This process is a chemical treatment that removes rust, scale, contaminants, and stains. Another strategy is to use mechanical grinding to remove surface rust and treat the metal with protective surface coatings such as zinc primer and red oxide primer.
In Summary
Does mild steel rust? The answer is yes, but this problem can be mitigated. Corrosion is a complex and degenerative process that affects the mechanical performance, properties, and useable life of construction and manufacturing components manufactured from mild steel, which can lead to cost and safety issues. This article has been a brief exploration of mild steel, its uses and properties, and the question of corrosion in this important engineering material.
More from AZoM: Which Alloys Are Prone to Corrosion, and How Can We Prevent This?
Further Reading and More Information
Velling, A (2020) Mild Steel – All You Need to Know [online] fractory.com. Available at:
https://fractory.com/what-is-mild-steel/