Surface treatment is an essential part of copper foil manufacturing for the electronics sector. These treatments are used for cleaning the copper foil, creating multiple or single layer thin film coatings for the prevention of oxidation or for improving the electro-chemical properties of the copper foil.
The chemical baths must be constantly monitored to ensure the highest quality surface treatments. Rigaku offers the NEX OL analyzer for satisfying these analytical needs for trend analysis of bath composition. Measurement of aqueous chemical bath solutions containing nickel, cobalt, molybdenum, zinc and indium is shown.

Instrument Configuratiom
| Model: |
Rigaku NEX OL Analyzer |
| Excitation: |
Direct with filters |
| X-ray tube: |
4 W Ag-anode |
| Detector: |
Silicon Drift Detector |
| Film: |
2 mil Kapton with 0.5 mil Teflon Coating (on wetted side) |

Figure 1. Rigaku NEX OL Analyzer
Sample Preparation and Presentation
By mixing powdered reagents for Zn, Ni, In, Mo and Co with the right water quantity, aqueous calibration solutions were prepared. Five calibrations standards were prepared for the Zn & In calibration and five standards were prepared for the Ni, Mo & Co calibration. All samples were measured in a static position using the auxiliary sample input loop.
Calibration and Measurement
Empirical calibrations were developed using aqueous samples. A summary of typical calibration is shown in Table 1.
Table 1.
| Component |
Concentration Range (mass%) |
RMS Deviation |
R2 Confidence |
| Zn |
750 - 1425 |
14.6 |
0.9958 |
| In |
140 - 320 |
4.2 |
0.9957 |
| Ni |
4500 - 5500 |
16.4 |
0.9973 |
| Mo |
780 - 1525 |
13.7 |
0.9976 |
| Co |
1275 - 1750 |
22.1 |
0.9805 |
Sample Recovery and Precision
A representative mid-concentration standard was chosen and analyzed against the empirical calibrations to demonstrate effective recovery and precision. The sample was analyzed 10 consecutive times in a static position. Typical performance results are provided below.
Table 2.
Sample ID: Zn-In Std3 Units: (ppm)
| Sample ID: Zn-In Std3 Units: (ppm) |
| Element |
Assay Value |
NEX OL Value* |
Standard Deviation |
RSD (%) |
| Zn |
1100 |
1118 |
16 |
1.5 |
| In |
220 |
222 |
3 |
1.2 |
* NEX OL Value reflects the average of the 10-repeat analysis.
Table 3.
| Sample ID: Zn-In Std3 Units: (ppm) |
| Element |
Assay Value |
NEX OL Value* |
Standard Deviation |
RSD (%) |
| Ni |
5200 |
5362 |
36 |
0.7 |
| Mo |
1100 |
1115 |
4 |
0.4 |
| Co |
1500 |
1547 |
19 |
1.3 |
Empirical Detection Limits (LLD)
The empirical method was used for determining the detection limits in a clean matrix containing no measureable elements. In the empirical method, 10 repeat analyses of a "blank" (deionized water) were taken and the standard deviation (a) was determined. The lower limit of detection (LLD) is then defined as 3a.
Table 4.
| Sample Type: DI Water Units: ppm |
| Component |
Empirical LLD |
Condition Count Time |
| Zn |
5 |
100 |
| In |
13 |
100 |
| Ni |
35 |
60 |
| Mo |
16 |
60 |
| Co |
19 |
60 |
Multi-Element Analysis
The Rigaku NEX OL system uses a high resolution semiconductor detector that achieves high sensitivity and resolution. It is possible to measure near-adjacent or adjacent elements with little or no peak overlap as these spectra show.
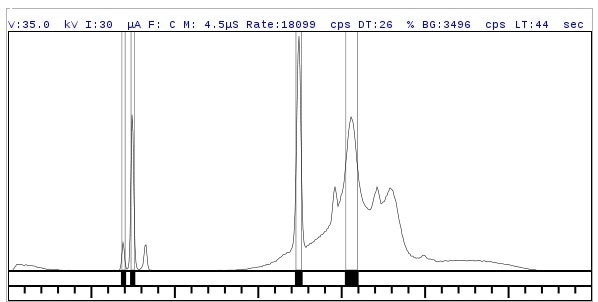
Figure 3.
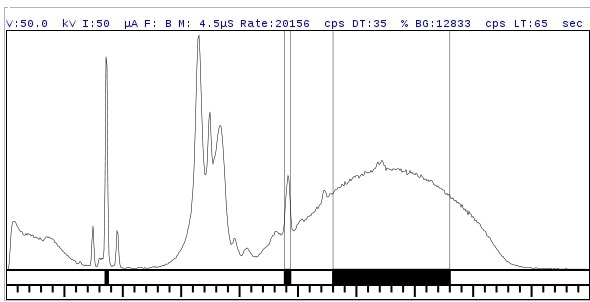
Figure 4.
NEX OL Key Features
The key features of NEX OL are:
- Trend analysis charting
- Real-time process control
- Capable of measuring elements Al to U, depending on application
- Robust Rigaku NEX QC+ optical kernel with SDD detector
- Industrial touch screen user interface
- Unique toolless flow cell design
- No dangerous radioisotopes
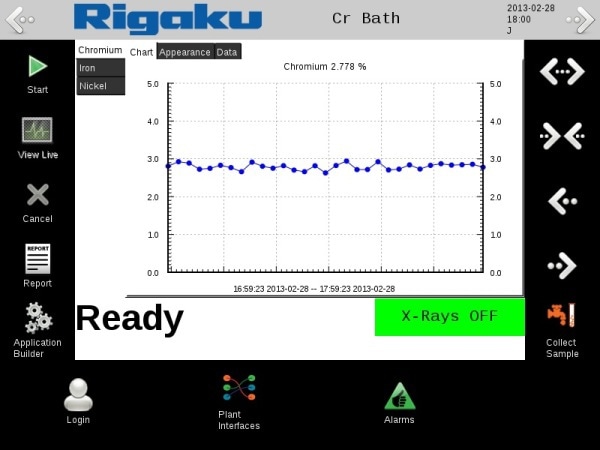
Figure 5.
Conclusion
The NEX OL offers high-throughput manufacturers a powerful, simple and versatile system for quantifying the elemental composition of their process stream. This study indicates that with stable samples, proper sample handling and proper calibration technique, the Rigaku NEX OL EDXRF can achieve excellent results in monitoring the concentration of chemical solutions used in the manufacturing of copper foil.

This information has been sourced, reviewed and adapted from materials provided by Rigaku.
For more information on this source, please visit Rigaku.