 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 11 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 11 2022In an article recently published in the open-access journal Polymers, researchers discussed the preparation of microporous wood-based activated carbon fiber and its utility for the removal of copper ions.

Study: Synthesis of Microporosity Dominant Wood-Based Activated Carbon Fiber for Removal of Copper Ions. Image Credit: andriano.cz/Shutterstock.com
Background
Several heavy metals are transferred into surface and subsurface water as a result of the rapid rise of industrial activity, posing substantial environmental dangers. Copper is a widely utilized metal in a variety of industries. These businesses generate a lot of wastewater and sludge with Cu2+ ions in it, which has a negative impact on the aquatic environment.
Heavy metals are poisonous and non-biodegradable, which affect human health and cause central nervous system disorders. As a result, developing effective solutions to clean Cu2+-polluted wastewaters before they are discharged into the environment is critical.
In the context of environmental protection, activated carbon fiber (ACF) is the most appropriate porous carbon material. ACF derived from liquefied wood (LWACF) is successfully synthesized and used as supercapacitor electrodes and prospective adsorbents. However, because of the difficulty in adjusting the pore size distribution (PSD), it has yet to be used in heavy metal adsorption.
The synthesis and use of mesoporous LWACF with ZnCl2 and H3PO4 activation are widely documented as matrix phase at the moment, but research into microporous LWACF for the adsorption of heavy metal is limited. Steam activation has become a popular approach for carbonaceous activation, and it can help carbon compounds increase their PSD.
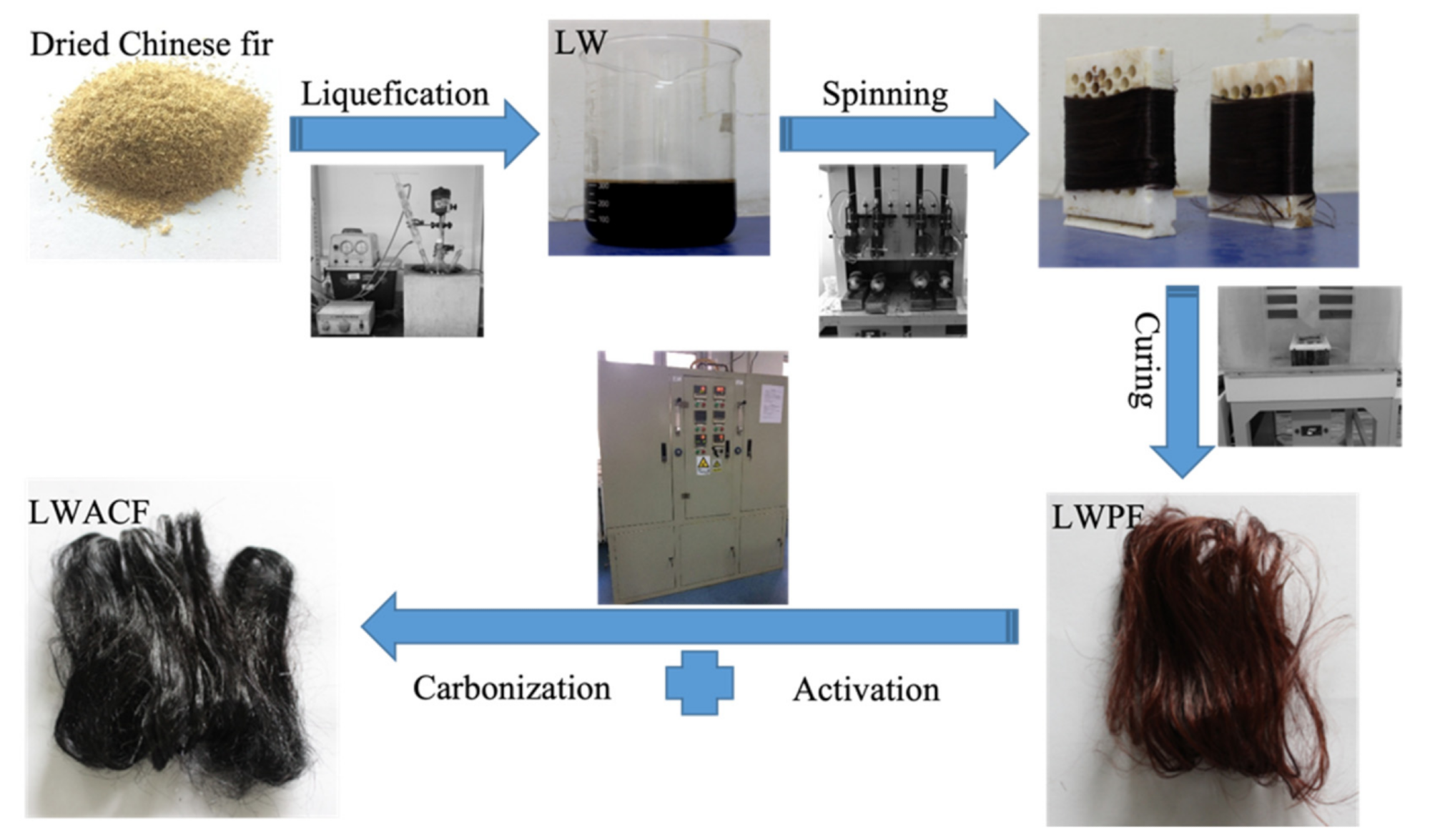
Synthesizing route of the series of LWACF. Image Credit: Jin, Z et al., Polymers
About the Study
In the present study, the authors presented the utilization of steam activation treatments to increase the specific surface area of ACF made from LWACF and to increase the pore size distribution. The effects of various preparation conditions on the pore architectures were also illustrated. Single-factor analysis studies were used to explore the parameters for LWACF synthesis, and static adsorption experiments were used to investigate the adsorption capabilities of this adsorbent.
Hook was pulverized and sieved to produce the precursor fibers, which were obtained through a number of procedures including melt-spinning, liquefaction, and curing. By adding a water stream combined with a nitrogen stream, the liquefied wood precursor fiber (LWPF) was activated at 800 °C for 60, 140, and 220 minutes.
Subsequently, they were cooled in a nitrogen stream to room temperature. X-ray photoelectron spectroscopy (XPS) measurements were used to analyze the surface chemistry, and scanning electron microscopy was used to examine the morphology of the prepared LWACF. At 196 °C, the porous structure of LWACF was investigated using a volumetric adsorption analyzer. The specific surface area (SBET) and average pore size (Da) were calculated using the Brunauer-Emmett-Teller (BET) model.
The t-plot approach was used to measure the micropore specific surface area (Smi), while density functional theory (DFT) was used to determine the mesopore volume (Vme), micropore volume (Vmi), and PSD. Cu2+ solutions with pH = 5.5 were made by dissolving 0.078125 g of CuSO4H2O in 1000 mL distilled water. An atomic absorption spectrophotometer was used to determine the amounts of heavy metal ions in the filtrates.
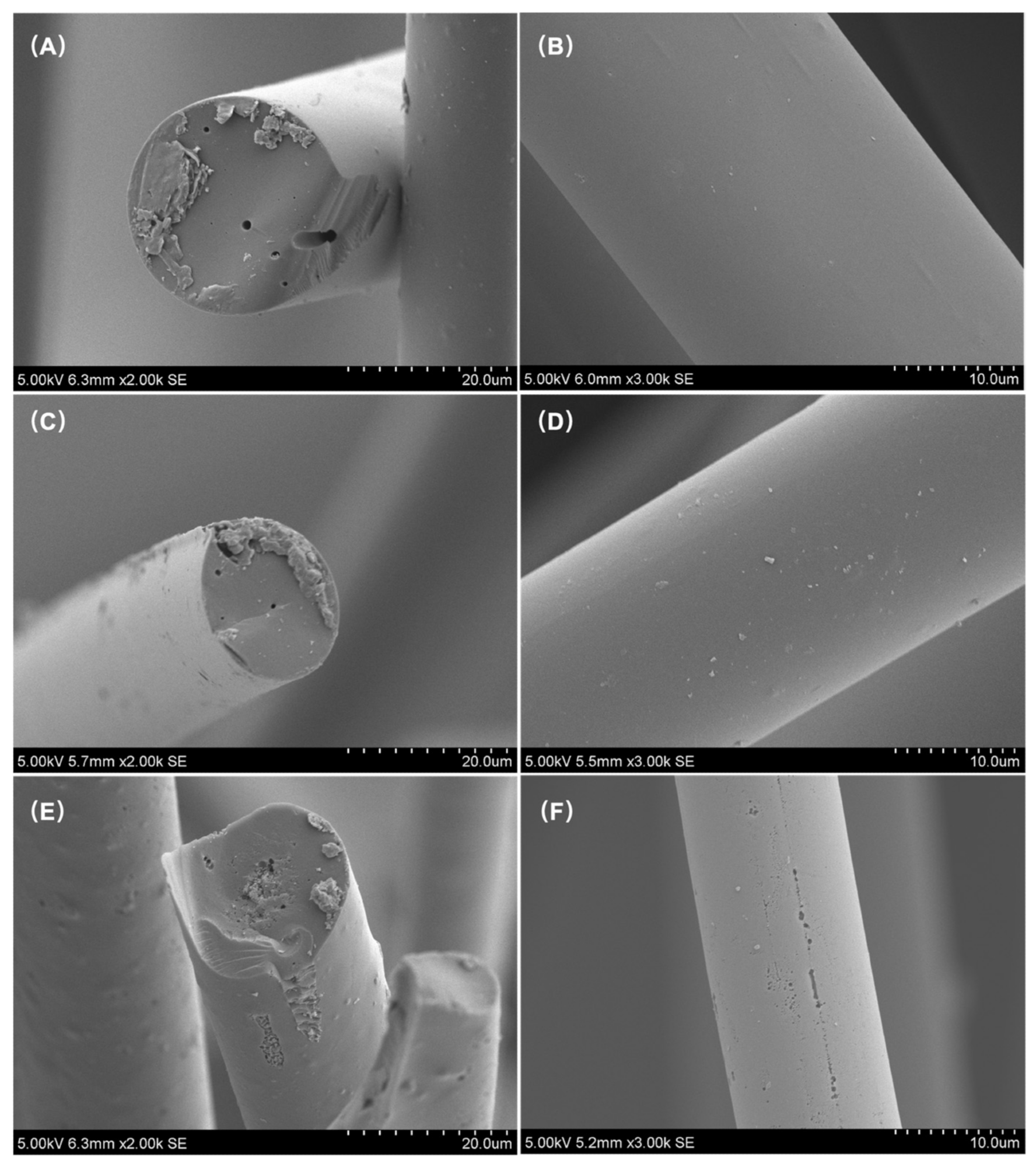
SEM images of LWACFs activated for 60 min (A,B), 140 min (C,D), and 220 min (E,F) at 800 °C. Image Credit: Jin, Z et al., Polymers
Observations
The average fiber diameter of LWACF reduced from 27.2 µm to 13.2 µm as activation time increased, while the specific surface area increased from 1025 to 2478 m2/g. Steam activation accelerated the development of microporosity while causing minimal pore widening. Due to an increase in the number of micropores and acidic-oxygenated groups, increasing the steam activation period exponentially increased the removal efficiency of Cu2+ at a constant adsorbent dose.
Furthermore, when the porous carbon fiber dose was increased from 0.1 to 0.5 g/L, the removal efficiency of Cu2+ was incremented from 55.2% to 99.4% for LWACF activated for 220 minutes at 800 °C. The average diameter of the fiber of LWACF decreased to 13.2 µm from 27.2 µm.
When the porous activated carbon fiber dose was raised from 0.1 to 0.5 g, the removal efficiency of Cu2+ increased from 43.6% to 66.1% for LWACF-140 and from 55.2% to 99.4% for LWACF-220. Controlled steam activation of the carbon surface introduced acidic functional groups like carboxyl and carbonyl, as well as made the carbon surfaces hydrophilic, which promoted strong attractive contacts between Cu2+ and the LWACF's active surface. Cu2+ removal efficiency was improved by increasing activation time while maintaining a fixed adsorbent dosage.
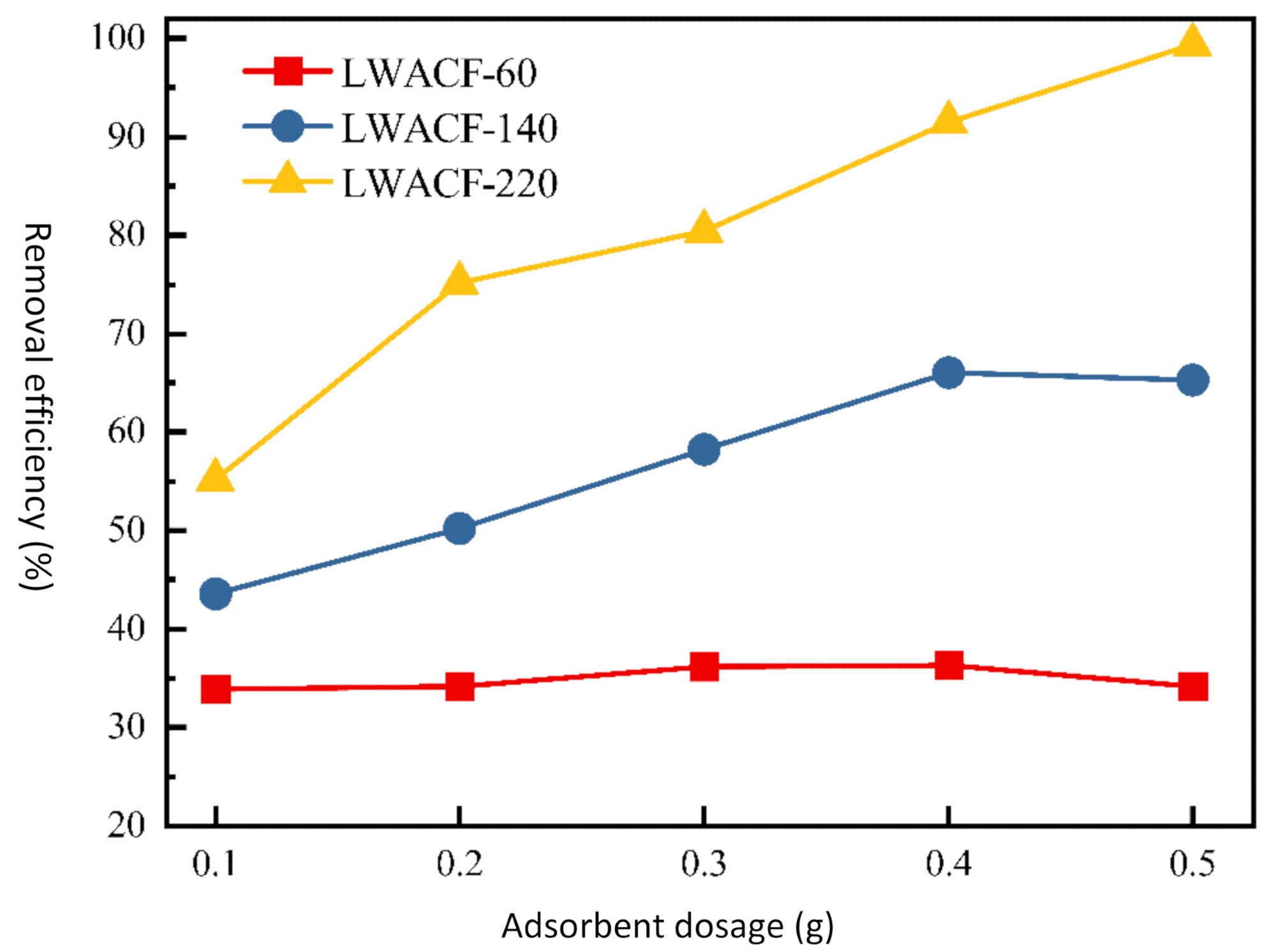
The removal efficiency of Cu2+ at a constant adsorbent dose for LWACFs with different activation times at 800 °C. Image Credit: Jin, Z et al., Polymers
Conclusions
In conclusion, this study presented the development of a series of LWACF samples that could be used to remove Cu2+ ions from aqueous solutions. The effects of steam activation pre-treatment were investigated in order to determine the adsorptive capabilities of the generated samples for heavy metal removal.
Longer activation times improved LWACF's Cu2+ ion removal efficiency. The maximum efficiency of elimination was 99.4%. The synthesized LWACF was found to be a highly effective adsorbent for Cu2+ ion-contaminated wastewater treatment.
Overall, the authors believe that the findings of this study will encourage the widespread use of activated carbon fiber made from biomass in the field of environmental protection.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Jin, Z., Zeng, Z., Hu, S., et al. Synthesis of Microporosity Dominant Wood-Based Activated Carbon Fiber for Removal of Copper Ions. Polymers 14(6), 1088 (2022). https://www.mdpi.com/2073-4360/14/6/1088