Oct 5 2010
Researchers led by University of Massachusetts Amherst chemist Vincent Rotello have demonstrated that they can deliver a dormant toxin into a specific site such as a tumor for anti-cancer therapy, then chemically trigger the toxin to de-cloak and attack from within.
It holds promise as a “complex and sophisticated” synthetic, therapeutic drug delivery system for living cells.
A paper describing the new host-guest chemistry approach by Rotello and colleagues, with Lyle Isaacs at the University of Maryland, appears in the current issue of Nature Chemistry.
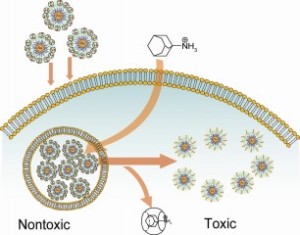 The image shows cellular uptake of cloaked gold (Au) nanoparticle-cucurbituril complexes and activation of toxicity by NH3 molecules inside the cell.
The image shows cellular uptake of cloaked gold (Au) nanoparticle-cucurbituril complexes and activation of toxicity by NH3 molecules inside the cell.
As Rotello explains, “Supramolecular chemistry focuses on understanding what forces make molecules stick together, and using these forces to control the assembly of functional systems. This assembly process is much like Lego blocks, where bumps and dimples interact to hold biomolecules like DNA and proteins together.”
Specifically, Rotello and colleagues covered specialized gold nanoparticles with ligand or binding molecules (the bump) that made the particles toxic. These ligands, however, also can strongly bind to a hollow, bowl-shaped molecule (the dimple to which the bump sticks) called a cucurbituril that can make the particle non-toxic. When the gold nanoparticles are introduced into living cells, they lie dormant. The researchers then use another molecule that binds strongly to the dimple-shaped cucurbiturils, pulling them away from the gold nanoparticle so it becomes uncloaked and toxic.
“This triggered toxicity opens up new directions for controlled chemotherapeutics, where toxicity can be tuned by and directed through choice and amount of added activator,” Rotello says. “They would be capable of achieving higher levels of site-specific activity with reduced collateral damage to surrounding healthy cells.”