Scientists from Forschungszentrum Jülich, a research institute, have identified an unexpected strong bonding between two organic layers. These structures create a base for innovative electronic components made from organic semiconductors.
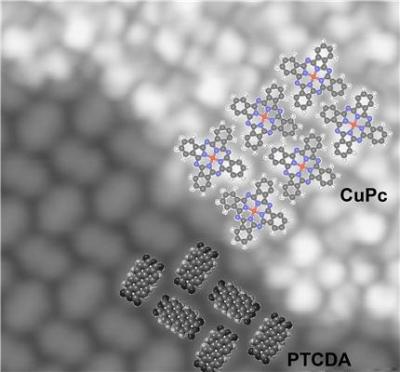 In the top right-hand of the image, a thin layer of copper phthalocyanine has become attached to a lattice made of PTCDA.
In the top right-hand of the image, a thin layer of copper phthalocyanine has become attached to a lattice made of PTCDA.
Organic semiconductors can be produced in flexible shapes and they are insensitive to external influences. In addition, the production of the organic semiconductors is cheaper. These materials are now extensively used as organic light-emitting diodes (OLEDs) in television sets and smartphones, as they consume very less power. However, the electronic properties of the organic semiconductors are still unknown to a large extent. Major interest of scientists is on the interfaces because the performance of components depends on the bonds formed with other metallic and organic conductors. If the bonds are stronger, then the transmission of electron from one material layer to the other will be better, thus enabling the light-emitting diodes or solar cells to generate more light or power. Nevertheless, organic materials do not typically produce strong bonds. Only when the materials come into contact with certain metals, stronger bonding is produced. This is termed as chemisorption.
Dr. Christian Kumpf from Forschungszentrum Jülich, remarked that their research has helped them to reveal the chemisorption property between organic layers for the first time. Jülich physicists have created a sandwich-like structure using copper phthalocyanine and a lattice made from PTCDA, an organic semiconductor material. The team has used ultraviolet photoelectron spectroscopy (UPS) to examine transfer of electrons between the organic semiconductors. In addition, low-energy electron diffraction (LEED) and scanning tunnelling microscopy (STM) have been utilized to exhibit the arrangement of the different molecules. The research shows that the organic molecules are transferred from one layer to the other in the same order with stronger bonding.