Researchers from the University of California, Riverside have developed a new method of using silica-coated sulfur nanoparticles coated to prevent the phenomenon called "polysulfide shuttling" in lithium-sulfur batteries.
 This is a schematic illustration of the process to synthesize silica-coated sulfur particles (Credit: UC Riverside)
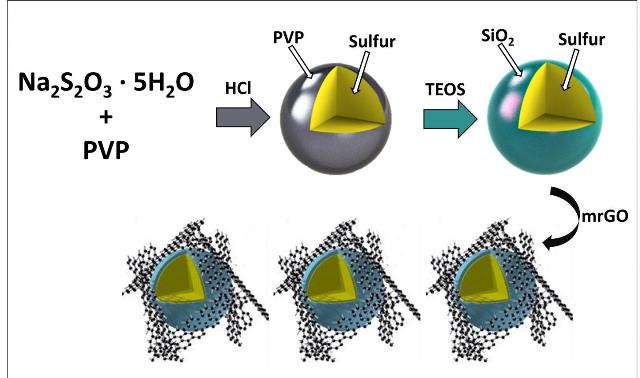
This is a schematic illustration of the process to synthesize silica-coated sulfur particles (Credit: UC Riverside)
Lithium sulfur batteries holds promise in electric vehicle applications as they are capable of producing energy that is 10 times more than that of conventional batteries. However, the generation of lithium polysulfides – products of reaction between lithium and sulfur, is one of the major drawbacks of these batteries. Upon dissolution, lithium polysulfides tend to move permanently towards the opposite electrode, resulting in the reduction of battery capacity.
The present work carried out by the members in Cengiz Ozkan's and Mihri Ozkan's research group involves development of a cathode material wherein polysulfides featuring a thin silica shell are trapped in silica cages. The research team constructed the trapping barrier using an organic precursor.
Our biggest challenge was to optimize the process to deposit SiO2 - not too thick, not too thin, about the thickness of a virus.
Mihri Ozkan
The team members, Robert Ionescu, Zachary Favors, Hamed Hosseini Bay, Jeffrey Bell, and Brennan Campbell, observed a significant increase in the performance of battery with the silica-caged sulfur particles. However, they realized the significance of improving silica-caged sulfur particles further due to the chances of silica shell breakage.
We have decided to incorporate mildly reduced graphene oxide (mrGO), a close relative of graphene, as a conductive additive in cathode material design, to provide mechanical stability to the glass caged structures.
Cengiz Ozkan
The researchers incorporated a flexible graphene oxide blanket to control silica and sulfur together during cycling. They also engineered the polysulfide-trapping barrier. The new generation cathode resulted in a significant improvement in the battery capacity when compared to the original design.
The design of the core-shell structure essentially builds in the functionality of polysulfide surface-adsorption from the silica shell, even if the shell breaks. Incorporation of mrGO serves the system well in holding the polysulfide traps in place. Sulfur is similar to oxygen in its reactivity and energy yet still comes with physical challenges, and our new cathode design allows sulfur to expand and contract, and be harnessed.
Brennan Campbell
The research is featured in the Nanoscale journal with the title "SiO2 - Coated Sulfur Particles as a Cathode Material for Lithium-Sulfur Batteries." Further, the research team has been invited to publish the results in the Graphene-based Energy Devices special themed issue in RSC Nanoscale.
The Winston Chung Global Energy Center at UC Riverside funded this research.
References