Aug 25 2016
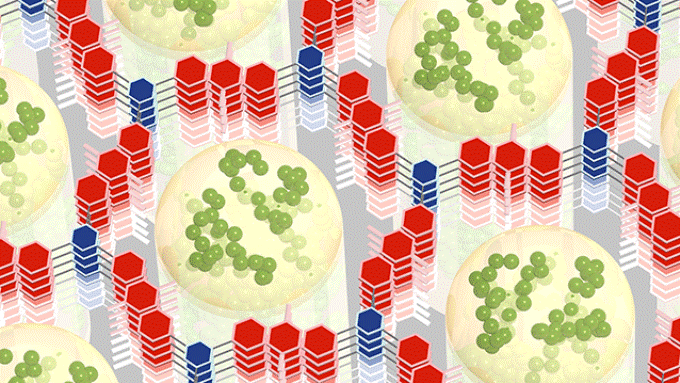 The best of both worlds. A new nanomaterial acts as both battery and supercapacitor: A conductive polymer (green) formed inside the small holes of a hexagonal framework (red and blue) works with the framework to store electrical energy rapidly and efficiently. (Credit: William Dichtel, Northwestern University)
The best of both worlds. A new nanomaterial acts as both battery and supercapacitor: A conductive polymer (green) formed inside the small holes of a hexagonal framework (red and blue) works with the framework to store electrical energy rapidly and efficiently. (Credit: William Dichtel, Northwestern University)
A research team headed by Northwestern University chemist William Dichtel has created a powerful new material that has the potential to eventually accelerate the charging process of electric cars and help enhance their driving range.
At present, the electric car relies upon a complex interaction between batteries and supercapacitors to provide the energy it requires to move. However this could soon change.
Our material combines the best of both worlds - the ability to store large amounts of electrical energy or charge, like a battery, and the ability to charge and discharge rapidly, like a supercapacitor.
William Dichtel, Chemist, Northwestern University
Dichtel and his team have integrated a COF - a strong, rigid polymer with plenty of minute pores ideal for storing energy - with a highly conductive material to develop the first altered redox-active COF that shortens the gap with other past porous carbon-based electrodes.
“COFs are beautiful structures with a lot of promise, but their conductivity is limited,” Dichtel said. “That’s the problem we are addressing here. By modifying them - by adding the attribute they lack - we can start to use COFs in a practical way.”
Additionally, altered COFs are commercially appealing, as they are made using low-cost, easily available materials, while carbon-based materials are costly to fabricate and produce in large scale.
Dichtel, the Robert L. Letsinger Professor of Chemistry at the Weinberg College of Arts and Sciences, presented the research findings recently at the American Chemical Society (ACS) National Meeting in Philadelphia. The research paper by Dichtel and co-authors from Northwestern and Cornell University was published by the journal ACS Central Science.
To exhibit the capabilities of the new material, the researchers constructed a coin-cell battery prototype device to power an LED for 30 seconds.
According to the researchers’ report, the material has exceptional stability, capable of 10,000 charge/discharge cycles. They also conducted detailed additional experiments to discover how COF and the conducting polymer, known poly (3, 4-ethylenedioxythiophene) or PEDOT, interacted together to store electrical energy.
The team created the material on an electrode surface. Two organic molecules self-assembled and condensed into a honeycomb-like grid, one 2D layer placed on top of the other. The researchers then deposited the conducting polymer into the grid’s holes.
Each hole measures just 2.3 nm in width, but the COF is full of these useful holes, forming a large surface area in a tiny space. A small quantity of the fluffy COF powder that can be filled into a shot glass, and weighs the same as a dollar bill, possesses the surface area of an Olympic swimming pool.
The altered COF exhibited a remarkable improvement in its ability to not only store energy but also to quickly charge and discharge the device. The material is capable of storing approximately 10 times more electrical energy than the unaltered COF, and it can obtain the electrical charge in and out of the device about 10 to 15 times quicker.
It was pretty amazing to see this performance gain. This research will guide us as we investigate other modified COFs and work to find the best materials for creating new electrical energy storage devices.
William Dichtel, Chemist, Northwestern University
The research was supported by the National Science Foundation, the Camille and Henry Dreyfus Foundation and the U.S. Army Research Office. The research was conducted at Cornell University, where Dichtel was a faculty member until this summer, when he moved to Northwestern.
The research paper is titled “Superior Charge Storage and Power Density of a Conducting Polymer-Modified Covalent Organic Framework.” Other authors of the paper are Ryan P. Bisbey, of Northwestern; Catherine R. Mulzer (née DeBlase, first author), currently at Dow Electronic Materials; and Luxi Shen, James R. McKone, Na Zhang and Héctor D. Abruña, of Cornell.