Mar 17 2017
Thanks to an innovative nozzle, scientists can now analyse more types of proteins while using fewer of the hard-to-get protein crystals. The nozzle can reduce protein consumption eightfold in serial X-ray crystallography experiments, as the team of inventors, headed by DESY scientist Saša Bajt from the Center for Free-Electron Laser Science (CFEL), writes in the journal Scientific Reports. The researchers used their novel nozzle to reveal hitherto unseen details of the structure of an RNA polymerase enzyme.
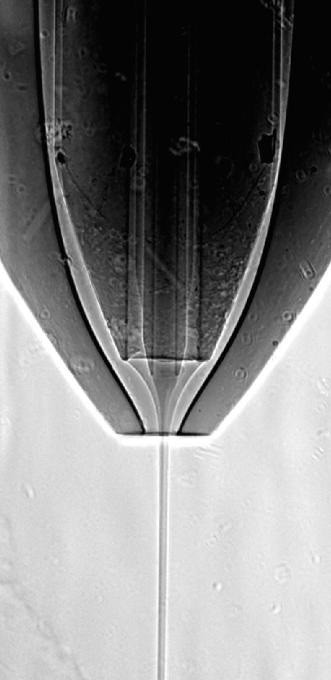 Radiograph of the working nozzle, showing the inner protein stream surrounded by the ethanol jet. (Credit: Dominik Oberthuer, DESY)
Radiograph of the working nozzle, showing the inner protein stream surrounded by the ethanol jet. (Credit: Dominik Oberthuer, DESY)
Scientists are interested in the spatial structure of proteins, as it reveals much about the workings of these biomolecules. This knowledge can lead to a better understanding of the functions of biomolecules and to tailored medicines. X-ray crystallography is the prime tool to solve protein structures. However, it requires crystals of the proteins under investigation. When X-rays hit these crystals, they are diffracted from the atoms to form a characteristic pattern from which the spatial structure of the crystal – and hence the protein molecules – can be calculated.
Many proteins do not like being squeezed into crystals as it contradicts their natural function. “Growing protein crystals is complex, the amount of protein that can be produced is often limited to few millionth of a gram and often only very tiny crystals can be obtained,” says Dominik Oberthür from DESY, main author of the report. With the extremely bright flashes of X-ray free-electron lasers even those micro crystals can be analysed, but usually thousands of diffraction patterns are needed to solve the protein structure. Since the delicate micro crystals are completely vaporised by the intense X-ray flash – after they delivered their diffraction pattern – a stream of fresh micro crystals is sent through the laser beam. This concept is known as serial X-ray crystallography and has enabled the analysis of many previously inaccessible proteins.
Still, even those micro crystals are hard to obtain, and only a fraction of them is actually hit by an X-ray flash, depending on the geometry of the crystal stream and the technical parameters of the X-ray laser. “The less crystals, the less protein material you need, the more feasible is the analysis,” emphasises Oberthür. Bajt's team conceived a new concept for a so-called double flow-focusing nozzle (DFFN) that greatly reduces protein crystal consumption. Usually, the protein crystals are injected with some carrier liquid (“buffer”) into the X-ray beam, using a special nozzle. To form a thin jet, the carrier liquid is accelerated by a fast stream of gas surrounding the liquid. But to form a stable jet, a minimum flow rate is needed, usually wasting most of the crystals in the jet.
To overcome these difficulties the team added ethanol (alcohol) as a secondary “sheath” liquid between the gas and the buffer. This leads to the sheath liquid being accelerated by the gas. The crystals in their buffer can then be injected as a very thin stream into the centre of the ethanol jet. “Before, the buffer with the crystals had to do two jobs: form a stable jet and carry protein crystals,” explained Juraj Knoška, a PhD student at CFEL and the University of Hamburg, who developed the nozzles. “Our approach separates these roles and uses the liquids that are best for the job.” Ethanol has ideal characteristics to form a very stable jet, which flows with just a fine stream of the crystal carrying buffer in the centre. This way, the flow rate of the buffer could be reduced from about 40 micro litres (millionths of a litre) to just 2 micro litres per minute. Also, the fine, stable stream of nanocrystals can be kept precisely overlapping with the small beam of the X-ray laser. In addition the reduction in overall flow-rate enhances the quality of the diffraction patterns and the rate at which crystals are actually hit by the X-ray flashes.
“Not only do we reduce crystal consumption, but our double flow-focusing nozzle also makes the use of the X-ray source more efficient by increasing the rate at which we collect high-quality diffraction patterns”, says Bajt. “Moreover, using the sheath liquid allows us to investigate proteins in buffers that couldn't be injected before. Our concept widens the spectrum of biomolecules that can be analysed.” Her team tested the new nozzle at the X-ray laser LCLS of the SLAC National Accelerator Laboratory in the US. The scientists teamed up with different groups to solve the structures of various proteins.
“Together with the group of Nobel laureate Roger Kornberg from Stanford University, we could solve the structure of the enzyme RNA polymerase II at room temperature for the first time,“ explains Oberthür. “Since crystallography at room temperature is a prerequisite to study structural dynamics in detail, this opens the door for future time-resolved studies or 'molecular movies' with this important system.” The new device was also used to analyse two other enzymes, a membrane bound hydrogenase and a dioxygenase as well as naturally occurring protein nanocrystals, from the protective cocoon of a specialised virus (Cydia pomonella granulovirus, CpGV).
The double flow-focusing nozzle also does away with another practical problem of this form of jet injection: Usually, at the edge of conventional nozzles, buffer material, protein and water ice crystals aggregate over time to form dripstone-like features. The same frequently happens at the bottom of the catch tank below the nozzle. If these protein-ice stalactites and stalagmites grow into the X-ray beam, they do not only render the diffraction pattern useless, their reflections can be so strong that they destroy the detector. So, every now and then, experiments need to be suspended to remove the protein-ice dripstones. “The sheath liquid in our nozzle prevents formation of such unwanted structures. The double flow-focusing nozzle enabled stable experimental conditions for many hours,” explains Oberthür.
“In all experiments the nozzle worked extremely well,” summarises Bajt. “We could reduce the number of interruptions from ten to zero in a shift, and we expect that experimental stations at other X-ray lasers and at synchrotron light sources like DESY's PETRA III can also benefit from the advantages of our device.”
The Arizona State University, the Cornell University, the University of Minnesota, the Technical University of Berlin, the Charité Universitätsmedizin Berlin, the Hauptmann-Woodward Medical Research Institute, the University of Nova Gorica, the Institute of Metals and Technology in Ljubljana, the Helmholtz-Zentrum Geesthacht, the University of Hamburg, and the Hamburg Centre for Ultrafast Imaging CUI were also involved in this research. CFEL is a cooperation of DESY, the University of Hamburg and the German Max Planck Society.