Nov 29 2017
Researchers have positively visualized terahertz radiation, commonly referred to as T-rays, using a crystal known as mayenite (Ca12Al14O33). Their technique ingeniously utilizes the rattling motion caused by the vibration of oxygen ions within the cage-like structures of the crystal.
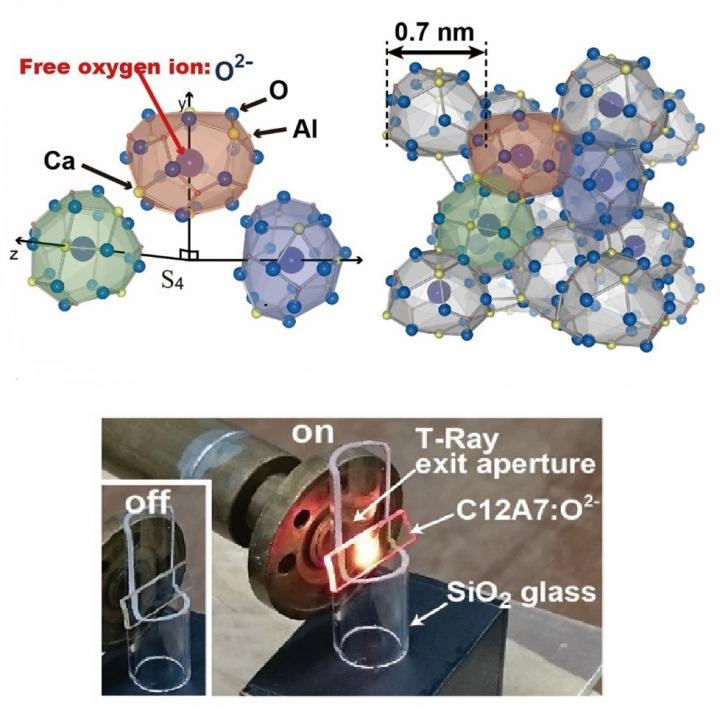 A schematic representation of the nanoscale cages. The oxygen anions randomly occupy one sixth of the cages. Bottom: A photograph of the emission of visible light at a terahertz radiation level of 0.21 and output power of 50 W (CREDIT - ACS Nano)
A schematic representation of the nanoscale cages. The oxygen anions randomly occupy one sixth of the cages. Bottom: A photograph of the emission of visible light at a terahertz radiation level of 0.21 and output power of 50 W (CREDIT - ACS Nano)
In recent years, there has been an increasing interest in creating practical devices based on terahertz technology. With wavelengths longer than infrared light, T-rays are said to be safer than conventional imaging systems. They are already used, for instance, at airport security checkpoints, and are starting to be employed more extensively in areas such as food inspection, medical screening and analysis of artworks. The visualization of terahertz light itself, however, has thus far proved difficult.
At present, Hideo Hosono of Materials Research Center for Element Strategy, Tokyo Tech and co-workers in Japan, Ukraine and the US have devised a simple method to convert T-rays to bright, visible light. Their research details have been published in ACS Nano.
First, the research involved beaming T-rays onto the mayenite crystal with a gyrotron. This created the vibration of oxygen anions, which collide with the inner walls of the cages within the crystal. Each cage has an outer diameter of 0.7 nm and an inner diameter of 0.4 nm.
The rattling of oxygen ions within the cages promotes upward energy conversion. Strong and frequent collisions of the oxygen ions induce electron transfer to neighboring empty cages. The excitation of the oxygen ions is key to the emission of visible light.
Hideo Hosono, Materials Research Center for Element Strategy, Tokyo Tech
Spectroscopy measurements established that the visible light originated from vibrations produced by the free-moving oxygen anions. The research team took care to exclude the possibility of other sources such as surface polarization and black body radiation as reasons behind the creation of visible light.
The research is an example of strategic study on functional materials under the Element Strategy initiative supported by Japan's Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Japan Science and Technology Agency (JST).
The crystal in our study is just composed of calcium, aluminium and oxygen, all of which are in the top five of the most abundant elements. So, it's one of the most inexpensive materials, at around 15 cents per kilogram.
Hideo Hosono
Regardless of its simplicity, Hosono says that the crystal has a number of exciting properties because of its nanostructure. Drawing on two decades of research, his group has already succeeded in showing that the material has exceptional catalytic properties for superconductivity and ammonia synthesis.
Best recognized for his pioneering research on iron-based superconductors, Hosono says that the present study marks a new research course. "Our group has been concentrating on the cultivation of new functionalities using abundant elements, but it's the first time for me to focus on ionic motion -- this is completely new," he says.
The findings could pave the way to the development of a T-ray detector, as no such conventional detector has yet been built.
Hosono added, "Right now, our material is good at detecting strong terahertz radiation. The challenge will be how to adjust the sensitivity."
His group has also reported that the oxygen anions can be replaced with hydrogen or gold anions within the cages. By employing these different anions, it may be possible to create detectors that produce different-colored light in future.