Feb 7 2019
For several years, researchers have been in search of an efficient and effective means to convert water into energy-storing fuels probably by splitting water into oxygen and hydrogen with the help of wind- and solar-powered electricity. To achieve this, they have been looking for catalysts that bring about these water-splitting reactions.
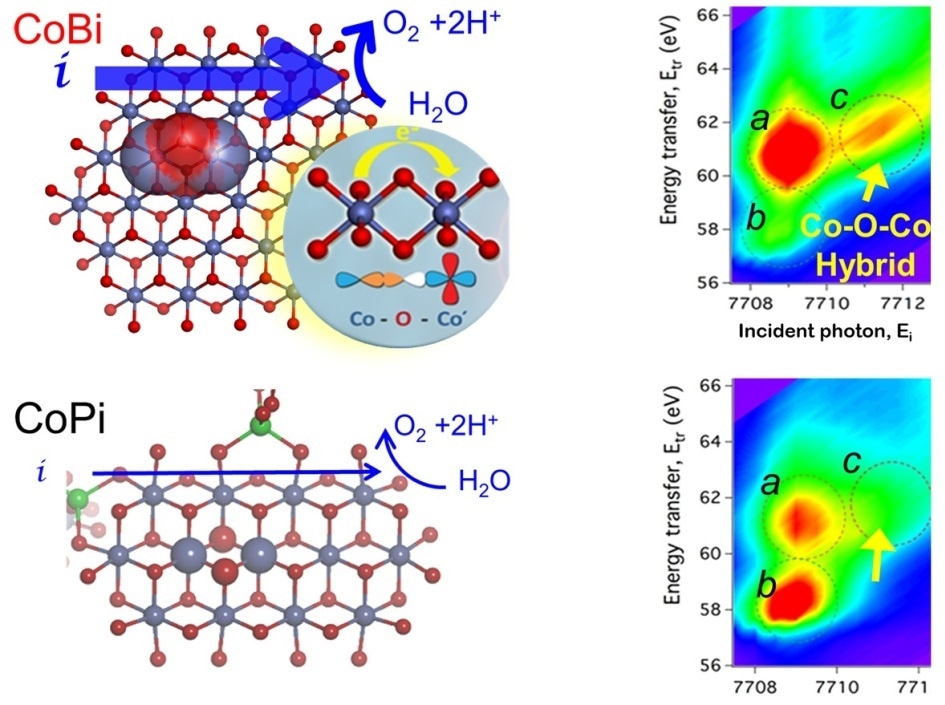 This graphic shows the “hybrid orbitals” in which atoms can share electrons in cobalt borate catalysts, making it a better water-splitting catalyst than cobalt phosphate. (Image Credit: Argonne National Laboratory)
This graphic shows the “hybrid orbitals” in which atoms can share electrons in cobalt borate catalysts, making it a better water-splitting catalyst than cobalt phosphate. (Image Credit: Argonne National Laboratory)
Scientists have known for quite a while that oxides of several metals, including the common iron oxide known as rust, have the ability to act as water-splitting catalysts, particularly when the atoms of the metal oxides are arranged into small clusters. However, the activity of these clusters, or domains, can greatly differ based on their structures.
In a new research of a related class of cobalt oxides, researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory wanted to find out why two similar catalysts with slightly different domain sizes behaved differently.
Our research team really wanted to understand why cobalt oxides that can be controlled to differ only in terms of their domain structure have such different water-splitting activities. Understanding this would provide a way to comprehend water-splitting catalysis for metal oxides more generally.
David Tiede, Chemist, Distinguished Fellow, Chemical Sciences and Engineering Division, Argonne National Laboratory
In the research, Tiede and his colleagues made use of Argonne’s Advanced Photon Source (APS) and Center for Nanoscale Materials (CNM), together with SLAC National Accelerator Laboratory’s Stanford Synchrotron Radiation Lightsource (SSRL), all DOE Office of Science User Facilities. They integrated a broad variety of various X-ray techniques and CNM’s electron microscopy abilities to investigate these domains at the atomic scale.
“The exciting thing about this research is that we’ve taken a truly multimodal approach that combines the power of soft and hard X-rays,” stated Tiede.
Last summer, Tiede and other scientists published a paper on the study in the Journal of the American Chemical Society.
Resonant X-ray techniques are a powerful tool to provide a wealth of structure and electronic information on metal oxide catalysts, particularly when they are structurally ill-defined.
Jung Ho Kim, X-ray Physicist, Argonne National Laboratory
Jung Ho Kim was also one among those authors who published the paper.
The scientists could demonstrate that the conductivity at the atomic scale controlled the differences in catalytic activity.
The scientists observed that when the cobalt oxide domains were formed in the presence of borate, electrons moved quite rapidly and smoothly throughout the material. However, when the cobalt oxides were formed in the presence of phosphate, electric charges were not able to move as easily.
According to Tiede, this difference is due to the fact that the cobalt atoms in the cobalt borate can share electrons with each other in what researchers call hybrid orbitals.
Essentially, you can think of the hybrid orbitals in cobalt borate as being like internet social media, whereas the orbitals in cobalt phosphate are like landline telephones. Information can travel more quickly through always-on networked connections.
David Tiede, Chemist, Distinguished Fellow, Chemical Sciences and Engineering Division, Argonne National Laboratory
Although cobalt phosphate has more active catalytic sites, the presence of the hybrid orbitals in cobalt borate makes it a better water-splitting catalyst when compared to cobalt phosphate. “Being able to move the charges to the active sites becomes the key factor in determining the efficiency of the catalyst,” Tiede stated.
Tiede and his team found something else unexpected while investigating the two cobalt oxides. Usually, the most difficult parts of catalysis are the bond-breaking and bond-forming steps, which are required in water-splitting process, but here, the most challenging part was getting adequate charge to the active sites. “Getting charges to the sites quickly enough is a key design parameter that we need to learn how to control,” Tiede stated.
It will be essential to combine charge mobility with water-splitting efficiency in order to develop a catalyst that can effectively convert water into electricity.
You can have the world’s greatest air conditioner, but if the wiring in your house is terrible, you’re not going to get it to work properly. The water-splitting site is doing a lot of complicated things, but if it’s not getting enough current, it’s not going to do very much.
David Tiede, Chemist, Distinguished Fellow, Chemical Sciences and Engineering Division, Argonne National Laboratory
The paper based on the work, titled “Resolution of electronic and structural factors underlying oxygen-evolving performance in amorphous cobalt oxide catalysts,” was published in the July 20 issue of the Journal of the American Chemical Society. Other Argonne authors included Gihan Kwon, Anil Mane, David Mandia, Sarah Soltau, Lisa Utschig, Alex Martinson and Hacksung Kim. Authors from SLAC included Hoyoung Jang and Jun-Sik Lee.
DOE’S Office of Science funded the study.