The pharmaceutical industry is looking to continuous processing to enhance production efficiency and product quality, in line with guidance from the regulatory agencies. However, in order to maximise the benefit of continuous processing, and obtain regulatory approval, it is necessary to establish the link between the processing parameters and the product attributes - something that is difficult to achieve due to the insensitivity of many traditional methods for powder and granule testing.
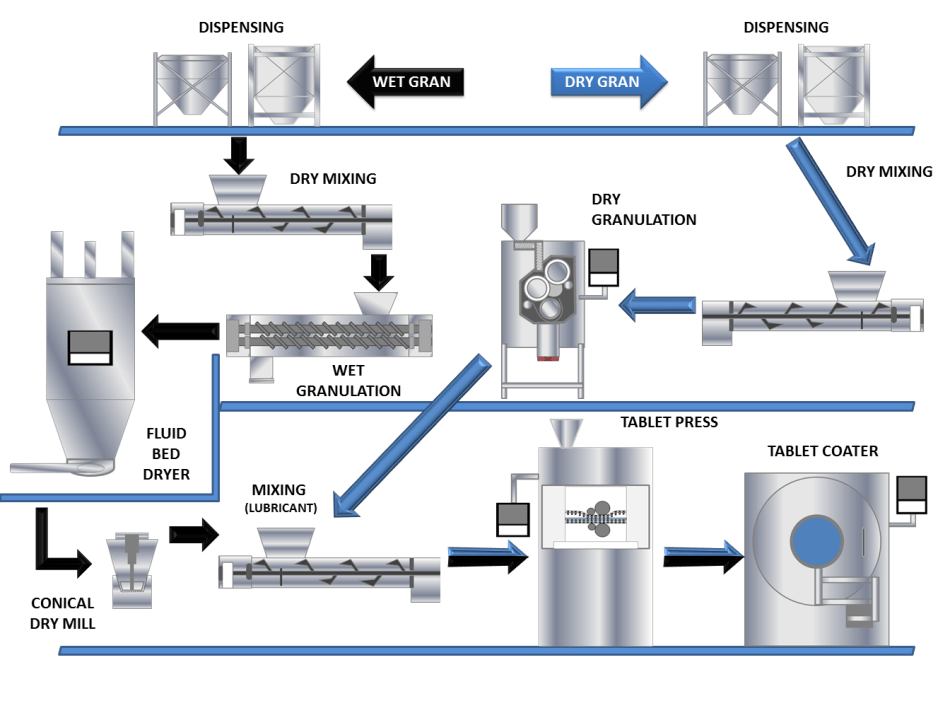
A study which summarises initial work between Freeman Technology and GEA has explored the relationship between granule properties and variation in formulation and processing parameters in a continuous manufacturing environment. To achieve this the capabilities of GEA’s ConsiGmaTM continuous high shear wet granulation and drying system and Freeman Technology’s FT4 Powder Rheometer® have been employed to continuously manufacture granules and then quantify the differences in their properties as a consequence of changing processing parameters and formulation.
The study was subsequently extended to include tablet manufacture, where correlations between granule properties and critical quality attributes of the tablets were identified, providing the information required for true Quality by Design (QbD).
To download your copy please visit the Freeman Technology website.