Nov 3 2020
Scientists from the National Institute of Standards and Technology (NIST) and their collaborators have shown a new room-temperature technique that could considerably decrease the concentrations of carbon dioxide (CO2) in fossil-fuel power plant exhaust. Such exhausts are one of the major sources of atmospheric carbon emissions.
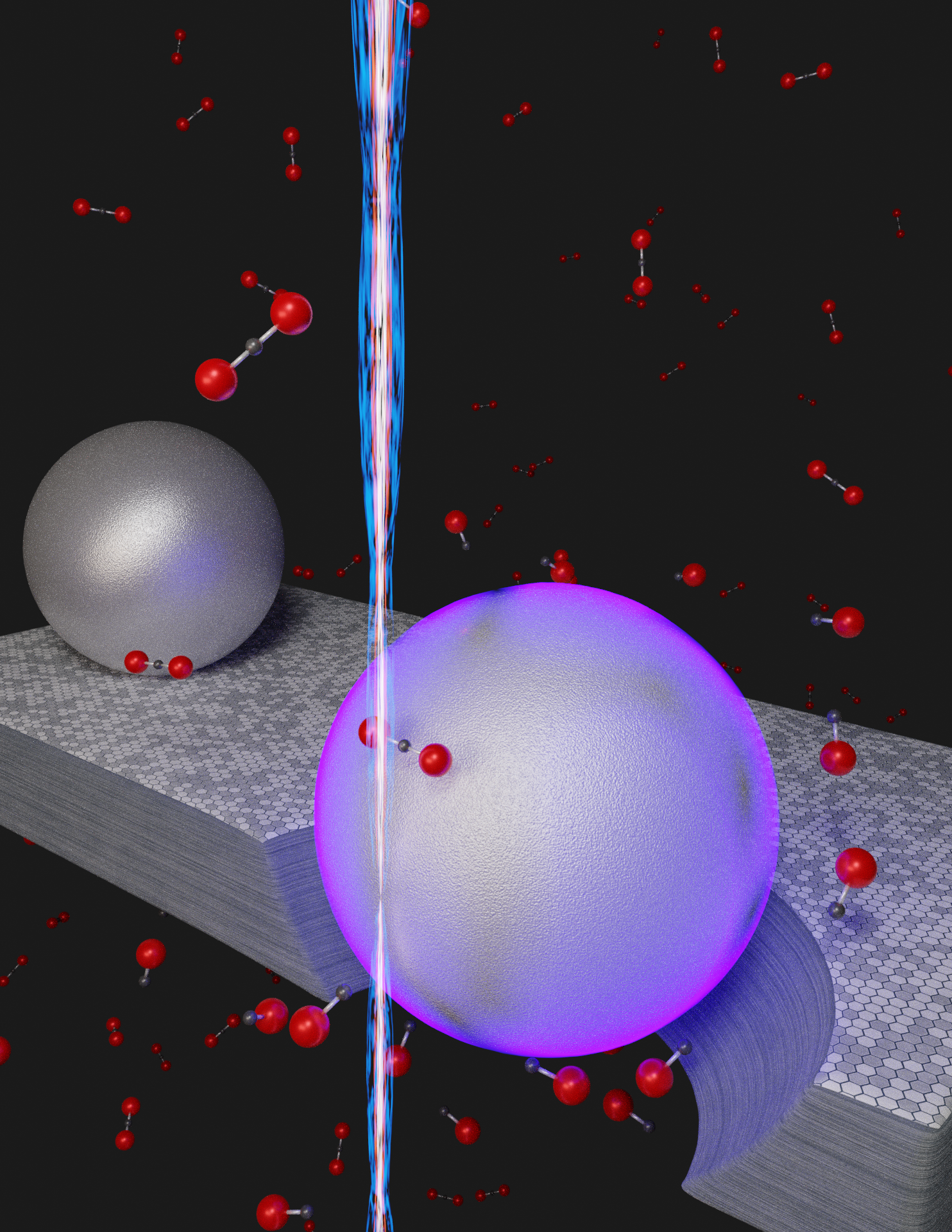 Illustration of a novel room-temperature process to remove carbon dioxide (CO2) by converting the molecule into carbon monoxide (CO). Instead of using heat, the nanoscale method relies on the energy from surface plasmons (violet hue) that are excited when a beam of electrons (vertical beam) strikes aluminum nanoparticles resting on graphite, a crystalline form of carbon. In the presence of the graphite, aided by the energy derived from the plasmons, carbon dioxide molecules (black dot bonded to two red dots) are converted to carbon monoxide (black dot bonded to one red dot. The hole under the violet sphere represents the graphite etched away during the chemical reaction CO2 + C = 2CO. Image Credit: National Institute of Standards and Technology (NIST).
Illustration of a novel room-temperature process to remove carbon dioxide (CO2) by converting the molecule into carbon monoxide (CO). Instead of using heat, the nanoscale method relies on the energy from surface plasmons (violet hue) that are excited when a beam of electrons (vertical beam) strikes aluminum nanoparticles resting on graphite, a crystalline form of carbon. In the presence of the graphite, aided by the energy derived from the plasmons, carbon dioxide molecules (black dot bonded to two red dots) are converted to carbon monoxide (black dot bonded to one red dot. The hole under the violet sphere represents the graphite etched away during the chemical reaction CO2 + C = 2CO. Image Credit: National Institute of Standards and Technology (NIST).
While the team was able to demonstrate this new technique in a small-scale, extremely controlled surrounding with dimensions measuring only nanometers (that is, billionths of a meter), they have already developed new ideas for upgrading the technique and rendering it viable for real-world applications.
Apart from providing a new, promising way of reducing the impacts of climate change, the chemical process used by the researchers may also bring down the costs and energy needs for creating various chemicals, including liquid hydrocarbons, utilized by the industry.
That is because the byproducts of the new method represent the building blocks for producing ethanol, methane, and other carbon-based compounds that are employed in industrial processing.
The researchers exploited a new source of energy from the nanoworld to activate a common chemical reaction that removes CO2. In this chemical reaction, solid carbon binds to one of the oxygen atoms in CO2 gas, decreasing it to carbon monoxide.
This conversion usually needs an enormous amount of energy in the form of high heat—that is, a temperature of minimum 700 °C, which is sufficiently hot to melt aluminum at standard atmospheric pressures.
But instead of using heat, the researchers depended on the energy collected from traveling waves of electrons, called localized surface plasmons, or LSPs for short. These LSPs surf on separate aluminum nanoparticles.
The researchers activated the oscillations of LSPs by triggering the nanoparticles with a beam of electrons that had a modifiable diameter. A narrow beam, measuring around 1 nm in diameter, struck the individual aluminum nanoparticles, while a beam measuring around a thousand times broader created LSPs among a huge set of the nanoparticles.
In the experiment performed by the team, the individual aluminum nanoparticles were deposited on a graphite layer; graphite is a form of carbon. This enabled the nanoparticles to transmit the LSP energy to the graphite layer. In the presence of CO2 gas, which was injected into the system by the researchers, the graphite played the role of plucking individual oxygen atoms from CO2, thus reducing it to carbon monoxide.
The individual aluminum nanoparticles were maintained at room temperature. In this manner, the researchers achieved a significant feat: eliminating the CO2 gas without the requirement for an extreme heat source.
Earlier techniques of removing CO2 had restricted success, because the methods used costly precious metals, required high pressure or temperature, or experienced poor efficiency. On the other hand, the LSP technique saves energy and, at the same time, uses aluminum—a low-cost, abundant metal.
While the LSP reaction produces a toxic gas—carbon monoxide—the gas instantly mixes with hydrogen to create essential hydrocarbon compounds, for example, ethanol and methane, that are frequently employed in industry, stated Renu Sharma, a NIST researcher.
Sharma and her collaborators, including investigators from the University of Maryland in College Park and DENSsolutions, in Delft, the Netherlands, published their results in the Nature Materials journal.
We showed for the first time that this carbon dioxide reaction, which otherwise will only happen at 700 degrees C or higher, can be triggered using LSPs at room temperature.
Canhui Wang, Researcher, National Institute of Standards and Technology and University of Maryland
The team selected a beam of electrons to activate the LSPs, because the electron beam can also be utilized to image structures in the system down to a few billionths of a meter. This allowed the researchers to predict the amount of CO2 that had been eliminated. They subsequently used a transmission electron microscope (TEM) to analyze the system.
Since both the amount of CO2 and the reaction volume of the experiment were very small, the researchers had to adopt unique steps to directly quantify the concentration of carbon monoxide produced. They achieved this feat by pairing a uniquely altered gas cell holder from the TEM to a gas chromatograph-mass spectrometer. This enabled the researchers to quantify parts-per-millions concentrations of CO2.
In addition, Sharma and her collaborators utilized the images generated by the electron beam to quantify the proportion of graphite that was etched away at the time of the experiment, a proxy for the amount of CO2 that had been removed. The team observed that the ratio of carbon monoxide to CO2 quantified at the outlet of the gas cell holder increased linearly with the quantity of carbon eliminated by etching.
Moreover, a large part of carbon etching—a proxy for CO2 reduction—took place close to the individual aluminum nanoparticles. This was confirmed by imaging with the electron beam.
Additional analyses showed that just around one-seventh of carbon was etched when the individual aluminum nanoparticles were not included in the experiment.
Restricted by the size of the electron beam, the researchers’ experimental system was very compact, measuring only around 15 to 20 nm across (the size of a tiny virus).
Hence, tpTo upgrade the system so that it could eliminate CO2 from the exhaust of a commercial power plant, a beam of light may offer a better option than a beam of electrons to trigger the LSPs, added Wang.
According to Sharma, a transparent enclosure comprising loosely packed carbon as well as aluminum nanoparticles could be positioned across the smokestack of a power plant. The LSPs would be activated by an array of light beams impinging on the grid. When the exhaust travels via the device, the light-activated LSPs present in the nanoparticles would offer the energy required to eliminate the CO2 gas.
The commercially available aluminum nanoparticles should be uniformly distributed to increase the contact with the carbon source and also with the incoming CO2 gas, noted the researchers.
The new study also proposed that LSPs provide a means for a range of other chemical reactions that currently need an enormous infusion of energy to proceed at normal pressures and temperatures using plasmonic nanoparticles.
Carbon dioxide reduction is a big deal, but it would be an even bigger deal, saving enormous amounts of energy, if we can start to do many chemical reactions at room temperature that now require heating.
Renu Sharma, Researcher. National Institute of Standards and Technology
Journal Reference:
Wang, C., et al. (2020) Endothermic reaction at room temperature enabled by deep-ultraviolet plasmons. Nature Materials. doi.org/10.1038/s41563-020-00851-x.