Freeman Technology is pleased to announce the release of a new application note “Using Powder Testing to Optimise the Processing Characteristics of Amorphous Solid Dispersions”.
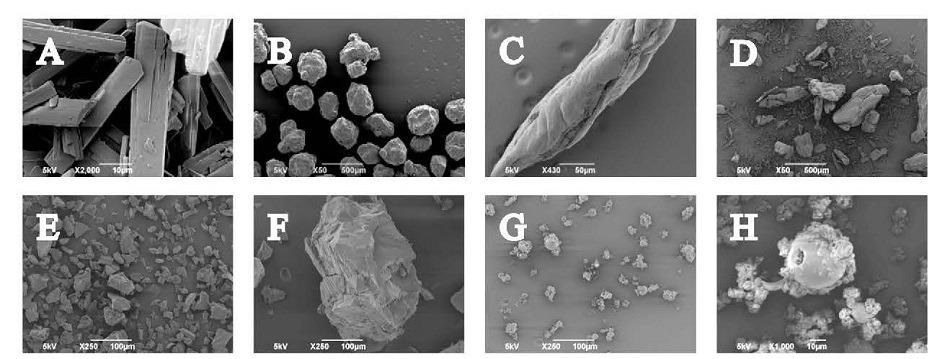
The use of amorphous solid dispersions (ASDs), in which the active(s) is dispersed in a hydrophilic, water-soluble excipient or matrix, is a widely recognized strategy for enhancing the bioavailability of poorly soluble drugs. However, the resulting materials are often associated with poor manufacturing efficiency. More specifically, flow properties and compressibility can be less than optimal for high throughput tableting. ASDs can be made by different processing routes including spray drying (SD) and hot-melt extrusion (HME) to produce compositionally identical dispersions with substantially different physical characteristics. There is therefore scope to control processability, within the constraints of increasing bioavailability and ensuring adequate stability.
This application note summarises work by researchers at the Bernal Institute, University of Limerick, Ireland to address the issue of characterizing ASDs to rationalize and predict differences in downstream processing performance. In a detailed study, the morphology and flow properties of ASDs produced by spray drying and hot-melt extrusion were measured and compared to identify differences with the potential to impact processing behavior.
Click here to download.