Mar 2 2021
Scientists recently invented a new approach for optimizing the electrocatalytic performance of palladium (Pd) in ethanol oxidation reaction, which is the main anodic reaction of direct ethanol fuel cells (DEFCs).
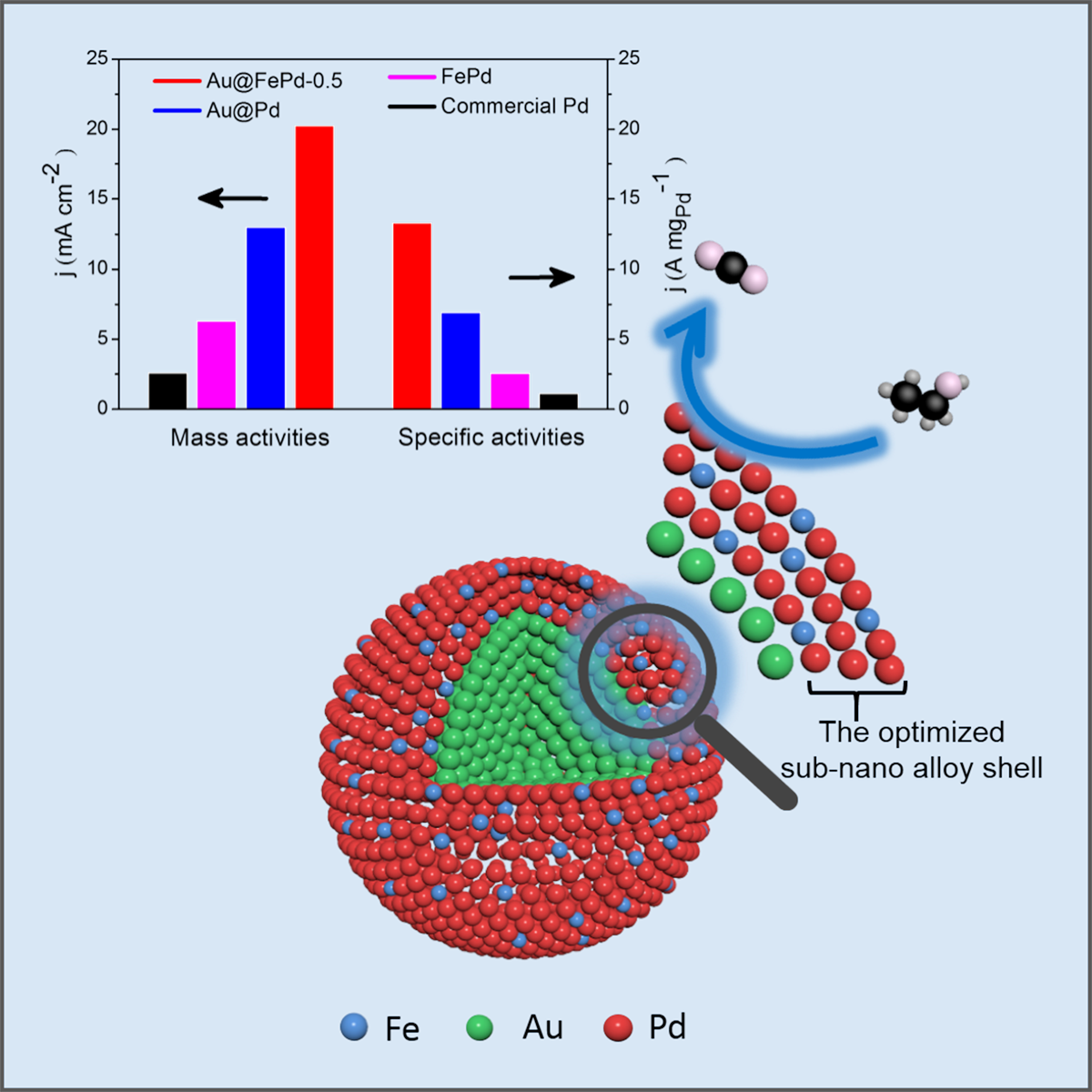 Combining core-shell construction with an alloying effect to boost the catalytic performance of palladium in ethanol oxidation reactions. Image Credit: Jun Yang.
Combining core-shell construction with an alloying effect to boost the catalytic performance of palladium in ethanol oxidation reactions. Image Credit: Jun Yang.
The new method devised by researchers at the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences and Nanjing Normal University provides an intelligent concept for fine engineering of the surface of electrocatalysts used in high efficiency energy conversion devices and beyond.
The study was reported in Cell Reports Physical Science on March 1st, 2021.
DEFCs that work with ethanol as fuel offer the benefit of low toxicity, high energy density and simple operation.
But the lack of robust, active electrocatalysts for anodic ethanol oxidation inhibits the pace of commercialization.
Essentially, Au@Pd nanoparticles including a core-shell construction exhibit higher stability and activity at the time of ethanol electro-oxidation compared to using Pd particles alone.
However, the larger lattice spacing of Au forms tensile strain in thin Pd shells, affecting their catalytic performance by boosting the absorption of toxic reaction intermediates on their surfaces.
A rational design that fully takes advantage of core-shell architectures and simultaneously inhibits the lattice tensile effect in Pd shells induced by Au cores would definitely be favorable for further improving their performance in the electro-oxidation of ethanol molecules.
Jun Yang, Study Corresponding Author and Professor, Institute of Process Engineering, Chinese Academy of Sciences
This concept was demonstrated by the team by combining alloying effects with core-shell construction to enhance the surfaces of Pd shells to realize high efficiency ethanol electro-oxidation.
Fe atoms were alloyed into thin Pd shells to compensate for their lattice expansion. Electrochemical assessments reveal that the core-shell Au@FePd nanoparticles synthesized through this method have the highest specific activity and mass activity to catalyze ethanol electro-oxidation ever achieved in an alkaline medium.
Next, we are going to optimize a number of parameters, e.g., the size of the core, the thickness of the alloy shell, and the composition of transition metals in alloy shells.
Jun Yang, Study Corresponding Author and Professor, Institute of Process Engineering, Chinese Academy of Sciences
Thus, the researchers believe they can synthesize more electrocatalysts with lower cost and higher efficiency to promote the development of direct alcohol fuel cells.
Journal Reference:
Liu, D., et al. (2021) Combining the core-shell construction with an alloying effect for high efficiency ethanol electrooxidation. Cell Reports Physical Science. doi.org/10.1016/j.xcrp.2021.100357.