New solvent mixtures developed by researchers from the Institute of Science and Engineering at Kanazawa University help break down the hard structure of plant cellulose for bioethanol production.
 Suitable solvents are needed to produce ethanol from cellulosic plant biomass. Researchers have found that liquid zwitterions are suitable for it, but it has been difficult to liquefy zwitterions, and the species have been strictly limited. Therefore, the solid zwitterions were liquefied by making deep eutectic solvents, which are liquids from solid-solid mixtures. The liquefied zwitterionic solvent was able to dissolve the cellulose. Image Credit: Kanazawa University.
Suitable solvents are needed to produce ethanol from cellulosic plant biomass. Researchers have found that liquid zwitterions are suitable for it, but it has been difficult to liquefy zwitterions, and the species have been strictly limited. Therefore, the solid zwitterions were liquefied by making deep eutectic solvents, which are liquids from solid-solid mixtures. The liquefied zwitterionic solvent was able to dissolve the cellulose. Image Credit: Kanazawa University.
Such new solvents tend to work under moderate conditions, exhibit less toxicity, and are more eco-friendly than existing solvents. Therefore, this study might pave the way for enhanced technologies to convert presently unused biomass to fuel.
Biofuels like ethanol produced from sugarcane or switchgrass might help reduce the dependence on non-renewable fossil fuels. But the process involved in producing biofuels generally involves breaking down cellulose from plants, which are composed of long polymer chains, into smaller sugar molecules.
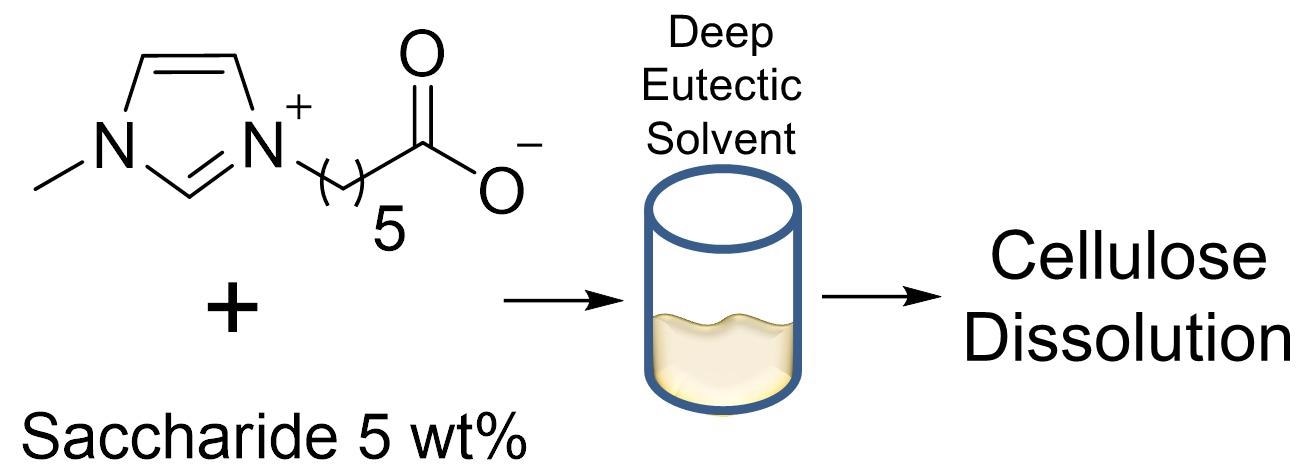
However, executing this process is not easy. Cellulose exhibits a hard hydrogen bonding network, making it extremely chemical resistant. Hence, the existing techniques for processing cellulose depend on toxic chemicals and severe reaction conditions.
Researchers from Kanazawa University have now utilized a unique class of molecules known as “zwitterions” to make novel solvents with the potential to dissolve cellulose. Zwitterions seem to be unique in that they can exhibit a positive or negative charge, but at various locations on the molecule so they become incapable of neutralizing each other.
These charges effectively break the hydrogen bonds that prevent the cellulose from being broken down.
Because almost all zwitterions are solid under normal reaction conditions, our experiments used eutectic mixtures.
Gyanendra Sharma, Study First Author, Faculty of Biological Science and Technology, Institute of Science and Engineering, Kanazawa University
A eutectic system is a blend of substances exhibiting a lower melting point than that of their constituents. This is achieved with the help of molecules featuring different structures so that regular crystals are more difficult to form.
During the experiments, four different zwitterions at various ratios were mixed by the researchers. They discovered 22 combinations in the liquid state having a melting point below 100 °C. Among these, two mixtures were also found to be highly effective at dissolving cellulose.
Our work shows that it is possible to replace many of the toxic chemicals used today with more environmentally friendly alternatives as we move towards a more renewable energy ecosystem.
Kosuke Kuroda, Study Senior Author, Faculty of Biological Science and Technology, Institute of Science and Engineering, Kanazawa University
This study illustrates the ability to utilize combinations of zwitterions to make mixtures that exhibit properties not exhibited by any molecule separately.
This study was financially supported in part by the KAKENHI (18K14281 from the Japan Society for the Promotion of Science) and the Leading Initiative for Excellent Young Researchers (from Ministry of Education, Culture, Sports, Science, and Technology-Japan).
In addition, this study was partially supported by the COI program titled, “Construction of next-generation infrastructure using innovative materials–Realization of a safe and secure society that can coexist with the Earth for centuries,” which is funded by the Ministry of Education, Culture, Sports, Science and Technology-Japan and Japan Science and Technology Agency, and Kanazawa University Sakigake project 2020.
Journal Reference:
Sharma, G., et al. (2021) Polar zwitterion/saccharide-based deep eutectic solvents for cellulose processing. Carbohydrate Polymers. doi.org/10.1016/j.carbpol.2021.118171.