The latest research by Japanese researchers in the journal Nature Scientific Reports focuses on the underlying concept of structure development during gas co-condensation and fast-rate Si nanorod production from a low-cost powder source via modified plasma flash evaporation.

Study: Silicon nanorod formation from powder feedstock through co‑condensation in plasma fash evaporation and its feasibility for lithium‑ion batteries. Image Credit: asharkyu/Shutterstock.com
The study reveals that Si nanowires/nanorods improve the recharging stability of lithium-ion batteries. However, sustainable large-scale synthesis of Si nanoparticles has not yet been achieved since it involves a costly chemical supply in general, as well as a low-rate and multi-step technique.
Importance of Silver
Structured materials can sustain the massive growth of silicon while retaining its high capacity. Because of their tiny size and asymmetric architectures, nanostructures such as Si nanoparticles, nanowires, and nanotubes have been employed to reduce the fracture.
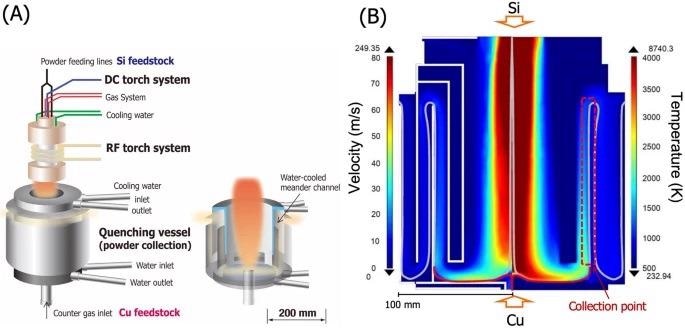
(A) Schematic illustration of the separate powder injection plasma flash evaporation (sPFE) apparatus. A hybrid plasma torch (DC and RF torches) is attached to the reaction chamber and a water-cooled gas quenching vessel is placed under the plasma torch. (B) Calculated plasma gas velocity and temperature distributions in the gas quenching vessel under the present sPFE condition. Image credit: Tanaka, A., et al., Nature Scientific Reports
Because of their distinctive chemical characteristics, silver nanoparticles (AgNPs) are rapidly being employed in a variety of industries, including medicine, nutrition, biomedical industry, consumer products, and other industries. They have positive thermal characteristics, as well as strong electrical conductivity and biological qualities.
Si vapor is delivered to a molten metal catalyst in VLS, and once supersaturated, Si grows as nanowires at the Si/metal catalyst contact.
Limitations
For the molten catalyst and Si vapor to coexist at distinct temperatures, traditional VLS necessitates a costly Si gas source, such as monosilane. Furthermore, because Si nanowires are formed from the melted catalyst, a thin coating of metal-activated carbon must be placed on the substrate ahead of time and heated to create nanosized droplets right before VLS.
Because of this limitation, VLS is a batch operation. Si nanowires must be manufactured continually from low-cost raw materials to be industrially viable.
Synthesis of Si Nanowire
Laser ablation, molecular beam epitaxy, SiO evaporation, solution-based techniques, and chemical vapor deposition have all been used to create Si nanowires (CVD). CVD, in particular, is desirable because it provides good control of the wire form and a high yield. Despite the differences across approaches, vapor-liquid-solid (VLS)18 is a common and important process for the formation of Si nanowires.
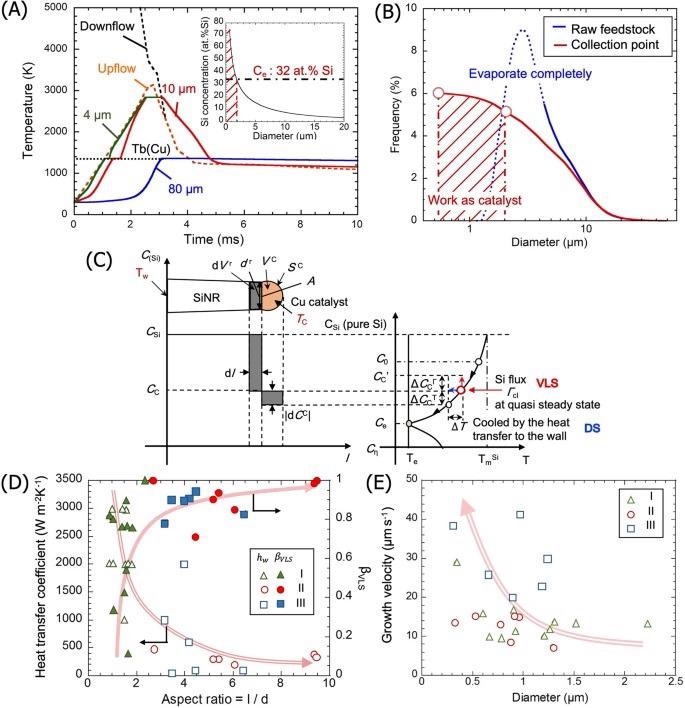
(A) Heating histories of Cu particles with different initial diameters. The inset is the Si concentration in the Cu droplets at the collection point. (B) Size distribution of the initial Cu particles and of the molten catalyst for DS mode. (C) Schematic representation of the mass transfer during nanorod growth, showing the Si redistribution at the growth interface (left) and the corresponding concentration in the Si–Cu binary phase diagram (right). (D) Relationship between the aspect ratio of the nanorods and the heat transfer coefficient and the VLS contribution. (E) The total growth velocity of nanorods as a function of rod diameter. Image credit: Tanaka, A., et al., Nature Scientific Reports
Utilization of Plasma Flash Evaporation
Plasma flash evaporation (PFE) is an industrial process widely used as a continuous manufacturing method for nanomaterials. PFE involves feeding raw granules into a plasma jet tube, which causes immediate vaporization followed by condensation, resulting in homogenous deposition and the development of nanoparticles.
Depending on the changed co-condensation route of the thermally active vapors, alloy nanoparticles and/or composite nanoparticles can be enhanced by combining various components in powder form.
Lithium-Ion Batteries
According to the research, lithium-ion batteries have been commercialized on a vast scale for the past few years. Lithium-Ion Batteries (LIBs) are made up of two electrode materials in which Li+ ions are reversibly intercalated back and forth, giving electrical power to an external circuit.
Because of the lower electrical resistivity associated with the connected metallic nanoparticles, LiB cells using these nanoparticles as the anode have increased capacity retention. As a result, PFE has the potential to produce nanosized particle materials from a power source. Furthermore, it may be designed to generate one-dimensional structures such as carbon nanotubes (CNTs).
Research Findings
For the research, Si nanorods have been produced by separate powder injection plasma flash evaporation (sPFE) using a hybrid plasma spray system. It was found that the Si powder is pulverized, whereas the Cu is spherical. These topologies are typical of the mechanical grinding and gas thermal decomposition preparation processes, respectively.
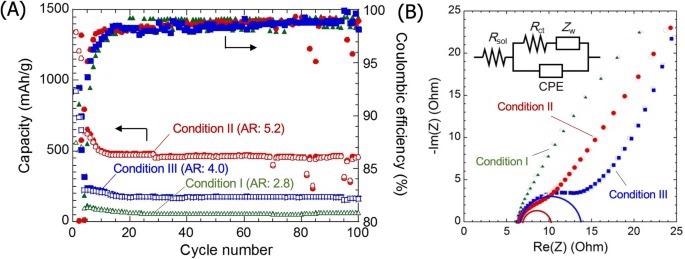
(A) Capacity and coulombic efficiency as a function of cycle number for cells prepared with Si nanorods of differing aspect ratio as the anode material. (B) Cole–cole plots for symmetric cells with different Si nanorods anode. Image credit: Tanaka, A., et al., Nature Scientific Reports
It is also noticed that Cu and Si nanoparticles are connected to the sidewalls of the rods, indicating that Si and Cu are continuously supplied during the formation of Si nanorods. While gas phase Si is given to one edge of the enzymatic particle during development, Si is completely saturated and repelled unidirectionally by the droplets on the other side.
Si concentrations in catalyst droplets are considerable, particularly for rods with a high aspect ratio. Furthermore, this suggests that the crystallization process at the catalyst/rod interface is primarily responsible for Si nanorod development, rather than the inclusion stage.
The growth period is primarily controlled by the cooling rate on the wall. Si is less likely to crystallize during the Cu droplet battle, and development begins only after Cu adhesion to the vessel wall. As a result, heat transmission to the vessel wall is the key limiting step in the formation of Si nanorods.
To conclude, an efficient synthesis of silicon nanorods formation from a powder feedstock using separate-injection plasma flash evaporation was demonstrated. It is expected that adjusting the temperature at the growth point to be high enough to preserve tiny Cu particles as a functional molten catalyst would result in longer Si nanowires with smaller diameters, ideal for battery anodes.
References
Tanaka, A., Ohta, R., Dougakiuchi, M., Tanaka, T., Takeuchi, A., Fukuda, K., & Kambara, M. (2021). Silicon nanorod formation from powder feedstock through co‑condensation in plasma fash evaporation and its feasibility for lithium‑ion batteries. Nature Scientific Reports. https://www.nature.com/articles/s41598-021-01984-y
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.