Reviewed by Alex SmithNov 26 2021
For the first-ever time, scientists at the University of Bayreuth and their collaborators from China and the United States have synthesized a carbon material that does not feature the exactly ordered structures of a crystal but is not amorphous either.
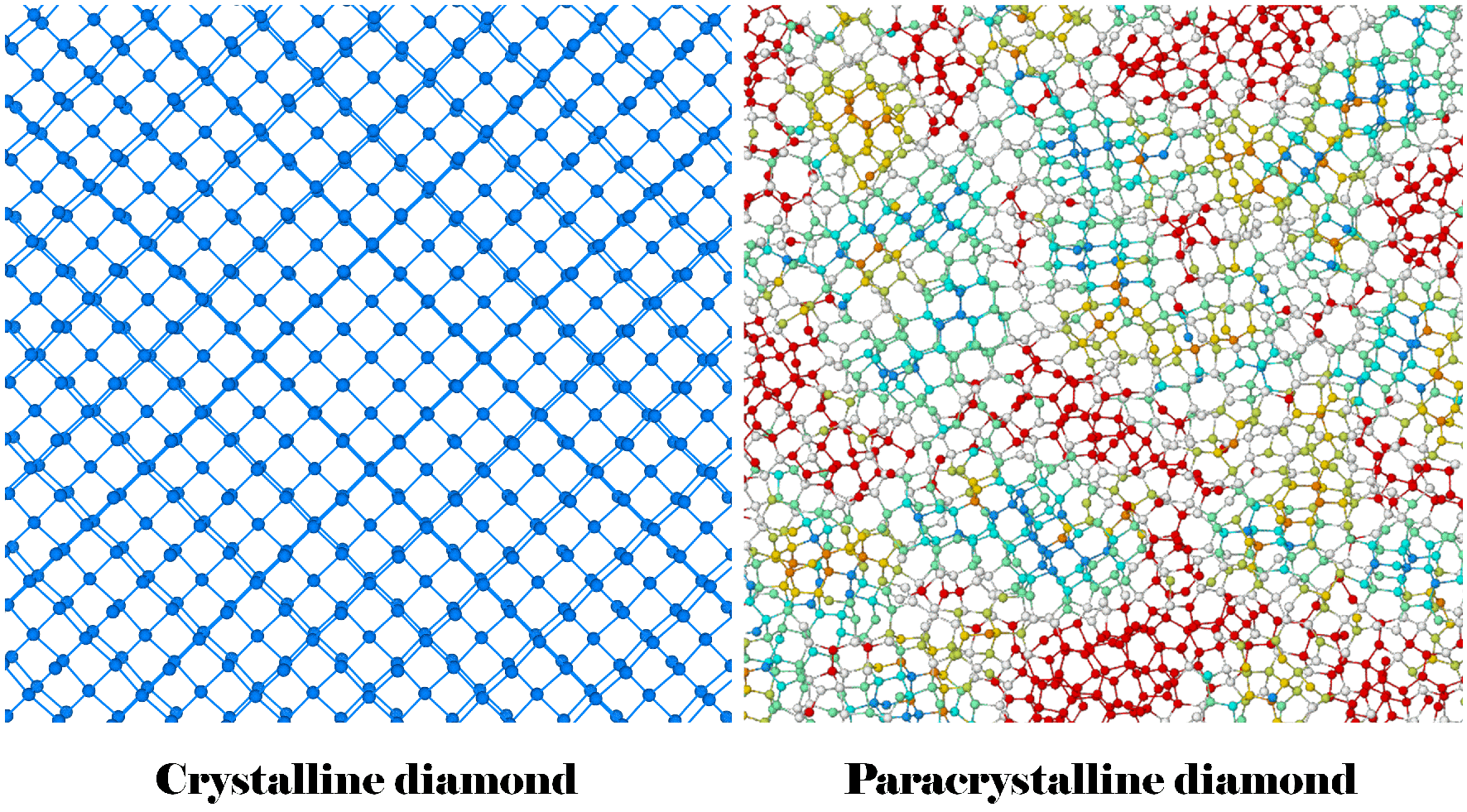 Structural comparison: crystalline diamond (left) and paracrystalline diamond (right). On the right, units of carbon atoms arranged in a cube shape are marked in turquoise; units of carbon atoms arranged in a hexagonal shape are marked in yellow. Irregular structures are marked in red. Image Credit: Hu Tang.
Structural comparison: crystalline diamond (left) and paracrystalline diamond (right). On the right, units of carbon atoms arranged in a cube shape are marked in turquoise; units of carbon atoms arranged in a hexagonal shape are marked in yellow. Irregular structures are marked in red. Image Credit: Hu Tang.
The new material is a paracrystalline diamond that has special optical, thermophysical, and mechanical properties. It provides essential clues for comprehending non-crystalline materials as well as for the targeted synthesis of other new carbon materials. The international group has reported its breakthrough in the journal Nature.
Diamond is an exceptionally hard material that develops naturally under very high pressures in the interior of the Earth. It is made of carbon atoms in a three-dimensional crystalline lattice structure. Each carbon atom inside this structure includes four covalent bonds. The four electrons in such bonds are divided among the orbitals of the atom in a characteristic way.
Thus, the state in which the carbon atoms in a diamond are situated is also known as “sp3 hybridization.” Diamond occurs in several crystal forms, among which the best known are hexagonal diamond (HD) and cubic diamond (CD). But the synthesis of non-crystalline diamond has proven technically hard, restricting the understanding of its properties, structure, and synthesis mechanism.
A study group headed by Professor Dr Tomo Katsura at the Bavarian Research Institute of Experimental Geochemistry and Geophysics (BGI) has thus been pursuing the aim of synthesizing millimeter-sized non-crystalline diamond with the help of their recently developed ultrahigh-pressure method in a large-volume multi-anvil press (MAP).
At a pressure of 30 gigapascals and a temperature of over 1,300 °C, in the state of sp3 hybridization, the carbon atoms formed a large-scale non-crystalline structure in which regularly structured units can be determined.
The new material can be described as a paracrystalline diamond, which differs from all previously known structural variations of diamond. It has a non-amorphous structure in which the carbon atoms are arranged partly in cubes, partly in hexagons, and partly in irregular structures. The unusual physical properties of the new material are non-directional and expected to further advance the study of high-pressure materials.
Dr Hu Tang, Study First Author, Bavarian Research Institute of Experimental Geochemistry and Geophysics and Research Center, University of Bayreuth
The material we synthesized is a hermaphrodite: for the first time, it forms a bridge between crystalline and amorphous, i.e. completely disordered, structures. It will stimulate materials research to look specifically for other new materials in this intermediate range.
Professor Dr Tomo Katsura, professor of high-pressure geoscience at BGI.
Synthesis of a paracrystalline diamond was done on a high-pressure press at BGI. The study of its structures and properties involved both experiments under high temperatures and pressures complicated computer simulations.
Researchers of the Bayreuth worked closely with study collaborators in China and the United States, specifically with Dr Huiyang Gou at the Center for High Pressure Science and Technology Advanced Research in Beijing, Professor Dr Ming-Sheng Wang at Xiamen University, China, and Professor Howard Sheng at George Mason University in Fairfax.
Journal Reference:
Tang, H., et al. (2021) Synthesis of paracrystalline diamond. Nature. doi.org/10.1038/s41586-021-04122-w.