Cyanohydrins are organic substances consisting of hydroxyl and nitrile groups. They are utilized in industrial chemistry as raw materials for alcohols, acids, polymers and other compounds.
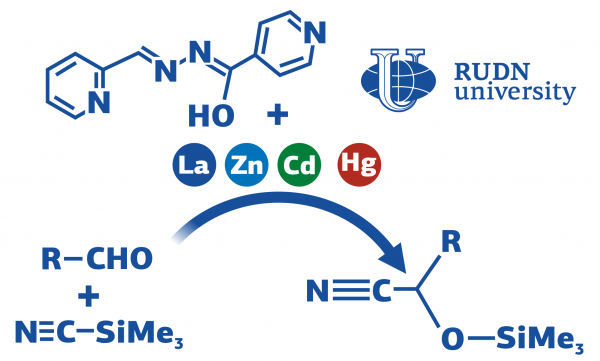
Image Credit: Russian Foundation for Basic Research.
One of the approaches through which cyanohydrins could be achieved is through the addition of prussic acid to aldehydes. The reaction is also possible without catalysts, but it gives a small yield of the product — nearly 20%.
To make the actual catalysts, there is a necessity for high-priced and risky reagents, and enough amounts are needed for the reaction itself. The chemists from Peoples’ Friendship University of Russia (RUDN) together with collaborators have illustrated that complexes of a few metals, the synthesis of which occurs under mild conditions, could act as efficient catalysts for this process.
Despite the wide variety of catalytic systems for cyanhydrin production, most of them have significant disadvantages — the use of harmful and expensive components and solvents, long reaction time, low yields, etc.
Evgenia Nikitina PhD, Associate Professor, Department of Organic Chemistry, Peoples’ Friendship University of Russia
Nikitina added, “Therefore, the development of a practical catalytic system for the efficient production of cyanhydrins from aldehydes under mild conditions remains a difficult task. We decided to test (investigate) several relatively easy-to-prepare and inexpensive metal complexes as potential catalysts.”
Four complexes of lanthanum, mercury, cadmium and zinc have been selected for catalytic reactions by the chemists. The same ligand was utilized to synthesize these complexes. Aldehyde, solvent and trimethylsilyl cyanide were added to the test tube along with the catalyst, and then the solution was blended.
The reaction’s route was tracked with the help of thin-layer chromatography. Once the reaction was over, the chemists from RUDN examined the consequent product using NMR spectroscopy.
The highly effective catalyst was a complex with lanthanum. In the presence of the catalyst at room temperature, the reaction went on for 6 hours. The final product’s output was around 96.3%.
Furthermore, the researchers analyzed which components of the lanthanum catalyst are accountable for its effectiveness and concluded that one of the most significant factors are donor-acceptor bonds and non-covalent interactions. The first — weaker — determines the molecule’s shape and stability, and the second — stronger — holds its very structure.
Compared to other coordination catalysts for similar aldehyde conversion, the lanthanum-based catalyst has better performance. The yield of the product is up to 96.3% in six hours at room temperature.
Fedor Zubkov PhD, Associate Professor, Department of Organic Chemistry, Peoples’ Friendship University of Russia
Zubkov added, “Moreover, the previously studied catalytic systems based on metal complexes require additives, a longer reaction time, a larger amount of catalyst, and higher temperatures, while the yield of cyanohydrins is usually lower.”
Journal Reference:
Huseynov, F. E., et al. (2021) Role of metal center and coordination environment in M-(Z)-N-((E)-pyridin-2-ylmethylene)isonicotinohydrazonate (M = LaIII, ZnII, CdII or HgII) catalyzed cyanosilylation of aldehydes. Polyhedron. doi.org/10.1016/j.poly.2021.115453.