.jpg) By Susha Cheriyedath, M.Sc.Reviewed by Skyla BailyJan 20 2022
By Susha Cheriyedath, M.Sc.Reviewed by Skyla BailyJan 20 2022A team of researchers recently published a paper in the journal Materials that demonstrated the suitability of using air and premixed hydrogen for microcombustion in an undulate channel to achieve flame stability.

Study: Combustion Characteristics of Premixed Hydrogen/Air in an Undulate Microchannel. Image Credit: peterschreiber.media/Shutterstock.com
Background
Technological advancements in the last few years have enabled the development of integrated system devices at the microscale level such as MEMS. These systems require power from external sources. Although batteries are often used as an external power source in such systems, typical limitations associated with using batteries have shifted the focus to the development of microcombustion-based alternatives.
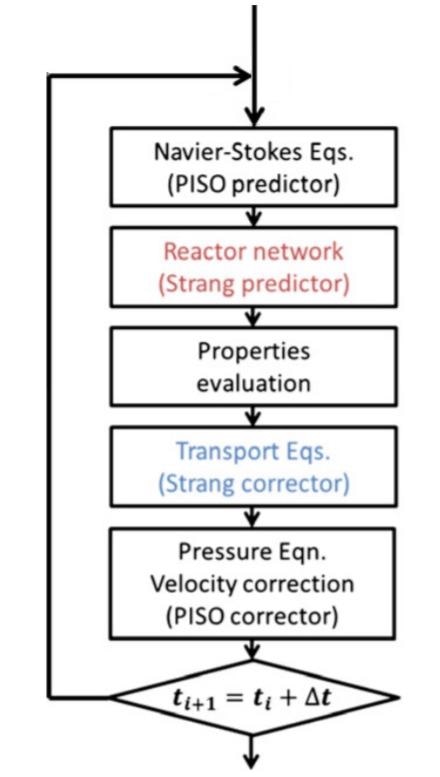
Numerical algorithm, adapted from Cuoci et al. Image Credit: Resende, P.R., Morais, L.C., Pinho, C. et al., Energies
However, obtaining a stable flame in microcombustion was difficult due to higher heat losses from the walls of the combustion chamber and insufficient time inside the microcombustor for complete combustion. In this study, researchers numerically investigated the combustion characteristics of one undulate microchannel in complex geometry using a mixture of air and hydrogen. The objective of the study was to observe the improvement in flammability at microscales by designing non-straight microchannels with the recirculation of reaction products.
The Study
The governing equations applied in this study for describing the microcombustion phenomenon include the conservation equations of energy, species mass fractions, momentum, and total mass. The thermal diffusion effect and Fick’s law were applied for calculating the diffusion velocities. The optically thin radiation hypothesis was assumed for modeling the radiative heat transfer. All these equations were implemented in the laminarSMOKE code. A thorough kinetic mechanism comprising 173 reactions and 32 species was utilized in the study to factor in the chemical reaction pathways of the premixed hydrogen combustion.
An undulate symmetric microchannel burner, representing the non-straight microchannel, was used in the study. The length and the maximum/minimum height of the undulated microchannel were 10 mm and 1.28/0.30, respectively. The height of the outlet and the inlet was kept at 0.8 mm for 2 mm length. The inlet temperature and the equivalence ratio were kept the same at Tin = 300 K and φ = 1.0, respectively, for all simulations. Similarly, the temperature profile was kept constant at the microchannel wall during every simulation.
A hyperbolic temperature profile was implemented at the burner walls, where the wall temperature rises from 300K at the inlet to 1300K at 1/5th of the channel length, and then remains constant for the rest of the channel. This temperature distribution profile was widely employed for mimicking the experimental profiles. A set of simulations was performed at various inlet velocities, ranging from 4 to 22 m/s. Two separate mesh resolutions were applied for verifying the independence of the results of this study from the earlier studies.
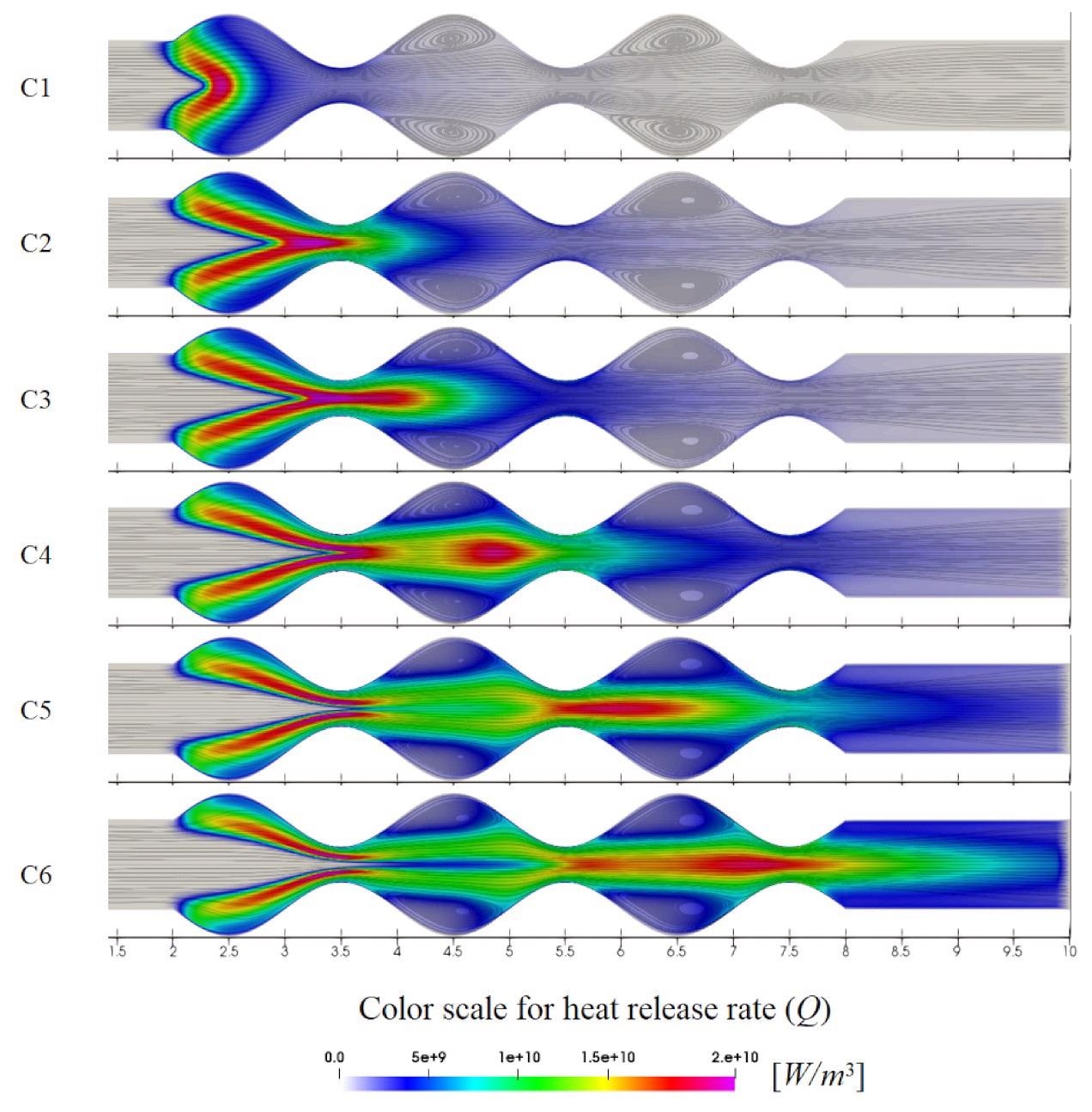
Contour maps for the streamlines superimposed on heat release rate distribution for different cases. Image Credit: Resende, P.R., Morais, L.C., Pinho, C. et al., Energies
Observations
Observations from the flame dynamics indicated that the flame becomes stable at the location where the values of Q distribution are maximum for every inlet velocity. The heat release rate was divided into two distinct regions for higher inlet velocities, and those regions became further stretched with the increasing inlet velocities. The vortices influenced the Q distribution for higher inlet velocities owing to the geometry of the burner, which squeezes the heat release through the middle of the burner.
The maximum temperature was higher for lower inlet velocities. However, the maximum temperature decreased with the increasing inlet velocity when the flame stretched downstream. Between the inlet velocities of 10 and 14 m/s, the flame splits into the two first cavity regions at the entrance of the burner, while for inlet velocities above 18 m/s, the flame divides into the three cavity regions of the undulated microchannel.
The flame structure became more stretched, and the maximum OH concentrations were observed further downstream of the microcombustion chamber as the inlet velocity increased.
Two different OH concentrations regions were observed at the entrance and downstream of the microchannel for inlet velocities higher than 14 m/s. The maximum flame temperature and the maximum OH value were reduced at higher inlet velocities due to the higher dispersion of the released energy. However, the higher dispersion of energy as the Q stretched downstream helped in maintaining an elevated flame temperature along the centerline of the burner. The rise in maximum values of Q is not sufficient for increasing the flame temperature.
The results indicated that the inlet velocity must be increased for shifting the loci of the maximum value for the temperature from the centerline to downstream in the undulated microchannel. The temperature rises gradually when the inlet velocity is increased and reaches the maximum value downstream. The flame attains the maximum temperature value at 3.15 mm and then decreases with the maximum values across the microchannel along the centerline in a stepwise manner.
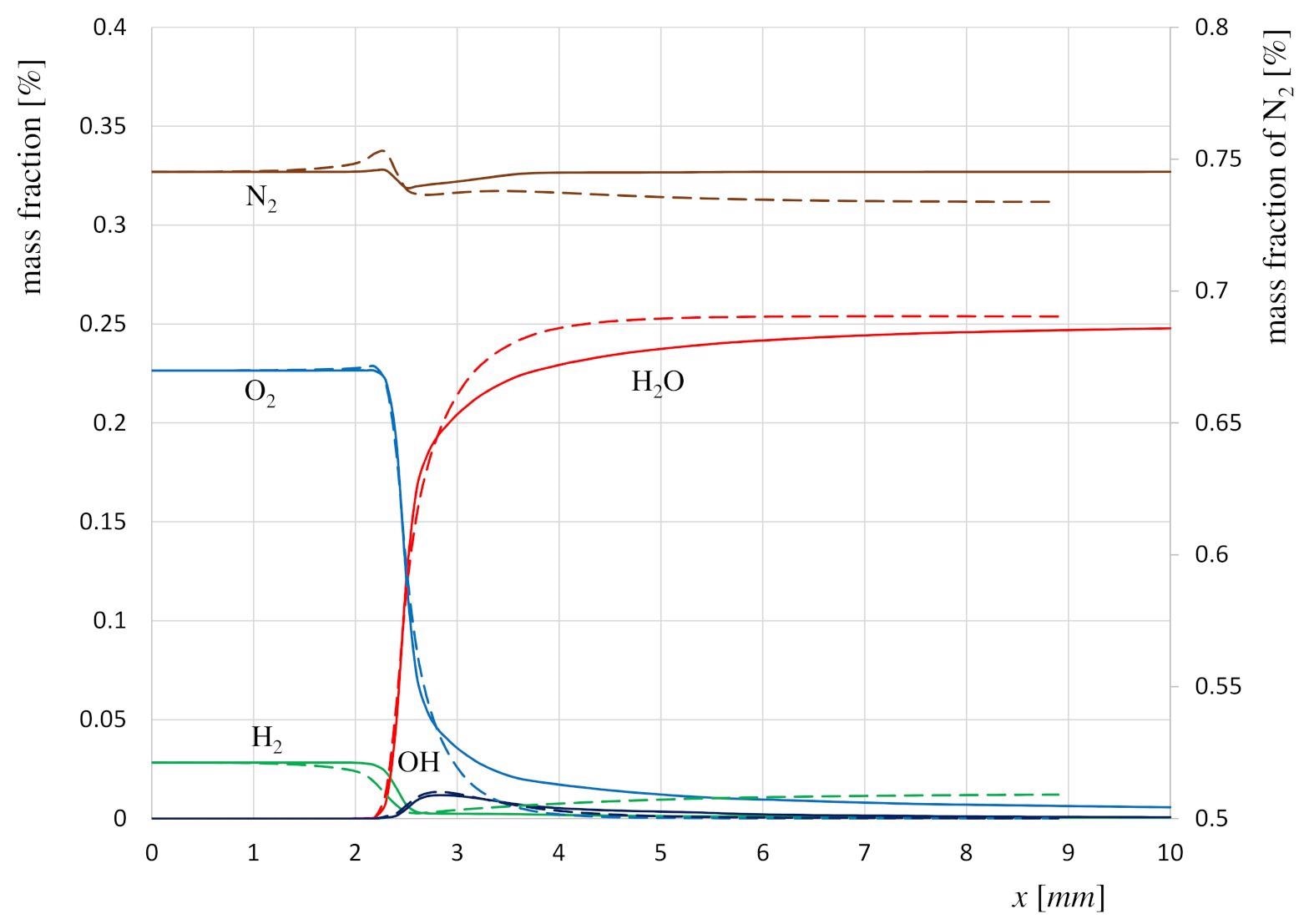
Comparison between the cases CH1 (continuum lines) and R1 (dashed lines) of the main species mass fractions in axial direction along the centerline. Image Credit: Resende, P.R., Morais, L.C., Pinho, C. et al., Energies
Significance
The study demonstrated a substantial reduction in heat flux losses through the microchannel walls, which is essential for achieving the required flame stability in microcombustion. Thus, the development of new and simpler geometries based on undulate shapes can act as a proper alternative to existing burners.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Resende, P.R., Morais, L.C., Pinho, C. et al. Combustion Characteristics of Premixed Hydrogen/Air in an Undulate Microchannel. Energies 2022, 15, 626. https://www.mdpi.com/1996-1073/15/2/626