Reviewed by Alex SmithFeb 7 2022
Colloids are nothing but microparticles present in a solution, which implies the particles are distributed uniformly. Crystals created from colloids are useful in an extensive range of applications like fuel cells, batteries, solar cells, sensors, and catalysts.
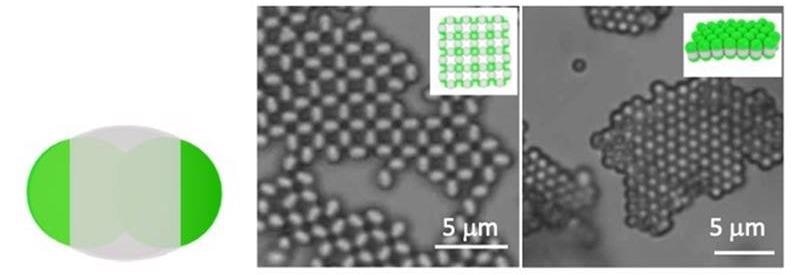 Schematic of a particle with distinct regions (left). The bright-field fluorescent images (center and left) and schematics (inserts) show selective crystallization via pole-to-pole or center-to-center interactions. Scale bars are 5 μm. Image Credit: David Pine, New York University.
Schematic of a particle with distinct regions (left). The bright-field fluorescent images (center and left) and schematics (inserts) show selective crystallization via pole-to-pole or center-to-center interactions. Scale bars are 5 μm. Image Credit: David Pine, New York University.
Researchers have looked for approaches to assemble these crystals into bigger structures with the help of bonding techniques that function in targeted directions. But this method seems to be exceedingly difficult.
A new strategy exploits the potential to make accurate regions with particular physical and chemical properties on the surface of the crystal particles. Researchers can use selective interactions between such regions on different particles to direct the crystal structure’s formation.
The Impact
This strategy is considered to be a robust method for programming the assembly of particles with preferred properties and geometry. The strategy consists of numerous advantages. It prevents the requirement for expensive and complicated chemical surface engineering.
The strategy also helps build materials ranging from simple chains to membrane-like structures, and can readily tune the properties and structures of the materials without having to make a new material from scratch each time.
Summary
Generally, the formation of colloidal crystals occur from a mixture of big and small particles. Attractive forces between the bigger particles drive the small particles away. The bigger particles squeeze jointly into highly tight packings that ultimately result in crystal formation.
This process of squeezing has been impacted by the composition of the smaller particles. Researchers point out that it is theoretically possible to make use of such forces to optionally place non-spherical particles inside a crystal lattice by making surface patches.
But researchers were not aware of this until now. Scientists have come up with a process for constructing hybrid particles made of clear polymer regions. In this process, tuning the surface charges on the particles favors interactions between a few regions.
This enabled a particular region of one particle to selectively interact with the same region on neighboring particles. The scientists utilized these interactions along with particle shape and size to control the bonding angles amongst the particles and accordingly the structural properties and features of the subsequent crystals.
Using advanced microscopy and computational methods, the scientists noted the concurrent formation of crystals having various structures in real-time. Such interactions are reversible. This method possibly enables direct reconfiguration of crystal properties and shifting between crystal structures.
This work was financially supported by the Department of Energy Office of Science. Partial assistance for one of the scientists was offered by the Donors of the American Chemical Society Petroleum Research Fund. The Zeiss Merlin field emission SEM was obtained with the help of the National Science Foundation.
Journal Reference:
Mingzhu, L., et al. (2020) Tunable assembly of hybrid colloids induced by regioselective depletion. Nature Materials. doi.org/10.1038/s41563-020-0744-2.