 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 16 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 16 2022In an article recently published in the journal ACS Applied Energy Materials, researchers discussed the development of high-performance silicon anodes in lithium (Li)-ion batteries with chitosan-grafted-gallic acid as a nature-inspired multifunctional binder.
Study: Chitosan-grafted-Gallic Acid as a Nature-Inspired Multifunctional Binder for High-Performance Silicon Anodes in Lithium-Ion Batteries. Image Credit: grebeshkovmaxim/Shutterstock.com
Background
Due to their superior energy density and cycle stability compared to current storage systems, lithium-ion batteries (LIBs) are widely used as energy storage devices in a wide range of applications. However, in order for these LIBs to be used in large-scale energy devices, they must increase their overall performance.
Silicon (Si) and its composites are considered pivotal anode materials for high-energy-density next-generation LIBs due to their high theoretical specific capacity and natural abundance. However, the large volume changes that occur during the repetitive lithiation/de-lithiation process result in the loss of electrical contact and the formation of a solid electrolyte interface (SEI), which limits Si's practical applicability. The logical design of the polymer binder is an effective way to protect the electrode's structure against substantial Si volume changes, hence improving lithium-ion battery cycle performance.
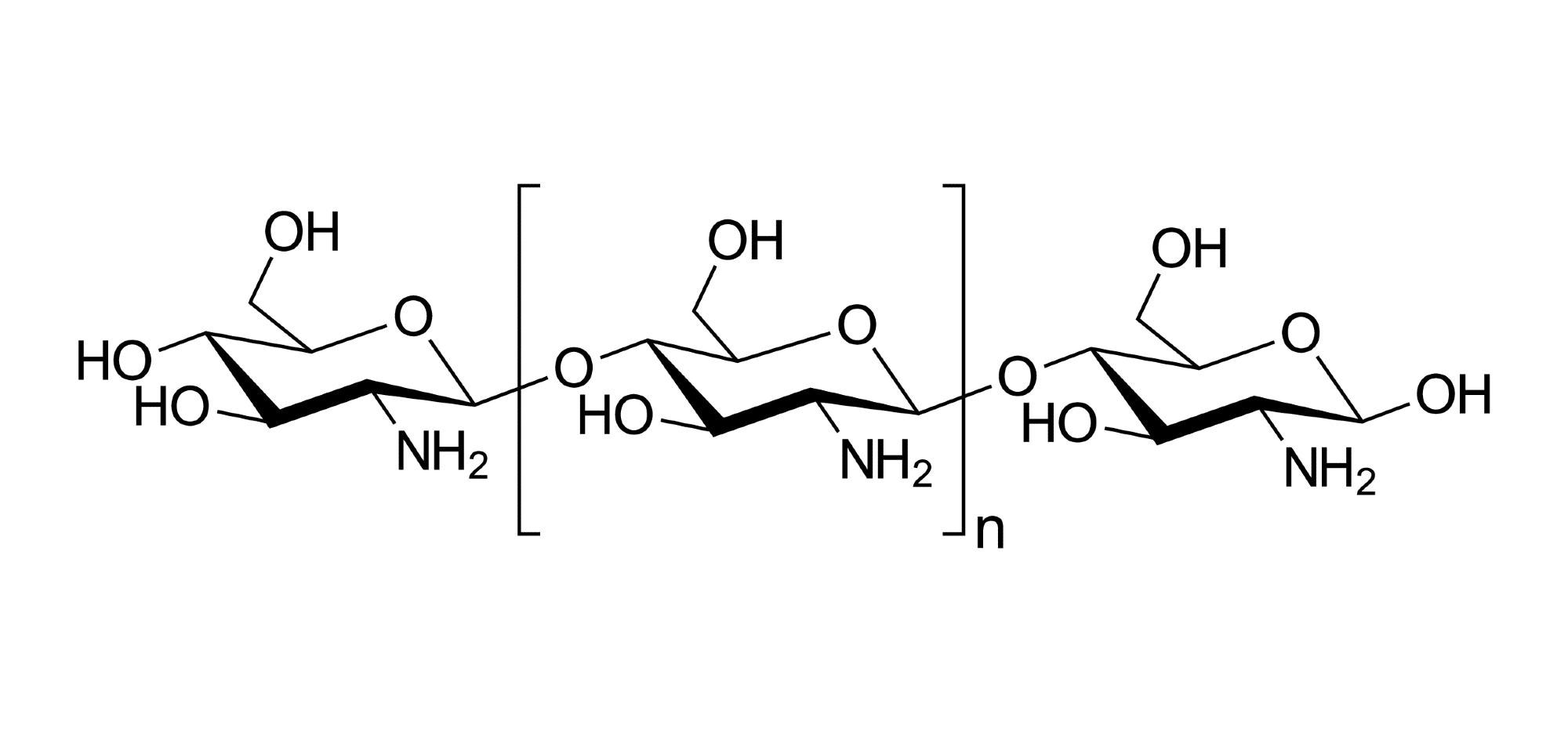
Furthermore, because of their structural-functional diversity, renewability, and abundance, biomass-derived materials are gaining traction as binder materials to be used in Si anodes for the fabrication of high-performance LIBs. Chitosan (CS) is a semicrystalline, linear polysaccharide generated by semi-deacetylation of chitin (poly (N-acetyl D-glucosamine), nature's second most prevalent polymer. However, no investigations on the application and usability of plant-derived gallol-containing polymers for LIBs like Si anode binders have been conducted to date.
About the Study
In the present study, the authors presented the development of an aqueous binder employing gallic acid (3,4,5-trihydroxybenzoic acid, GA), a plant-inspired sticky phenolic moiety that was grafted onto the marine-based polymer CS by a simple radical reaction.
By grafting GA, a plant-based material, onto the molecules of CS, a marine material, a new binder for Si anodes was prepared that could be dissolved in a non-acidic and aqueous solution. Through an H2O2/AA redox system-based free radical grafting procedure, the active hydrogen atoms of the -OH and -NH2 groups of GA were efficiently grafted onto CS.
More from AZoM: Photoresist Coatings and The Semiconductor Industry
GA (3,4,5-trihydroxybenzoic acid) was employed to increase the solubility of CS. CS-grafted-GA (CS-g-GA) was used to create an aqueous polymeric binder, in which GA is grafted onto the side chains of CS. This functionalization approach used simple reaction conditions and no hazardous chemicals, compared to traditional approaches that use EDC-mediated amide coupling reagents or activated ester reactions, lowering manufacturing costs and enhancing sustainability. The influence of the grafted GA content on the physical and electrical properties of the Si electrodes was investigated.
Observations
The chitosan-grafted-gallic acid, CS-g-GA, improved CS's water solubility and also improved its binding properties to Si. Si@CS-GA-100 had a high reversible specific capacity of 1868 mAh g-1 and capacity retention of 67% at a current density rate of 0.5 C after 350 cycles.
The CS-GA-100 electrode demonstrated enhanced cycling performance. Furthermore, the electrode retained a high-rate capacity of 2175 mAh g-1 at a higher current density of 5 C. The Si/Gr@CS-GA-100 electrode demonstrated excellent cycling performance at a current density of 0.5 C, with a capacity retention value of 67.2% when tested from the second to the 200th cycle. The reversible capacity of the Si/Gr@CS-GA-100 electrode was 965 mAh g-1 after the 200th cycle.
The CS-GA-100 binder electrode’s reversible capacity was estimated as 2331 mAh g-1 after 100 cycles at 0.2 C, and its corresponding Coulombic efficiency (CE) was 99%. This electrode had a capacity of 1512 mAh g-1 after 200 cycles when the current density was increased to 1 C and higher mass loading conditions were used. The CS-g-GA binders worked well with Si/Gr composite anodes too. Even after 200 cycles, these binders had a large capacity of 965 mAh g-1 at a current density of 0.5 C.
Conclusions
In conclusion, this study discussed the development of new binder materials using simple-to-handle, environment-friendly, sustainable, and low-cost biologically active chemicals. The prepared CS-g-GA binders were found to be highly suitable for Si anodes for the development of next-generation lithium-ion batteries. Si anodes made with the fabricated CS-g-GA binders had outstanding charge capacity, adhesion, rate, and cycle performance, and were able to accommodate Si volume expansion efficiently.
The authors observed that because of the strong adhesive ability of GA, the CS-g-GA generated was highly soluble in water and more efficiently accommodated the rapid volume change of Si electrodes during the lithiation and de-lithiation processes.
They also believe that the proposed binders have a lot of promise for use in commercial electrodes in the future.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Rajeev, K. K., Jang, W., Kim, S., et al. Chitosan-grafted-Gallic Acid as a Nature-Inspired Multifunctional Binder for High-Performance Silicon Anodes in Lithium-Ion Batteries. ACS Applied Energy Materials (2022).https://pubs.acs.org/doi/10.1021/acsaem.1c03791