Supercapacitors are considered to be an increasingly popular alternative to lithium-ion batteries, providing a longer lifetime and greater power density (several cycles where capacity is retained). A supercapacitor is esentially a cross between a regular capacitor (with high power discharge) and a battery (with high energy storage).
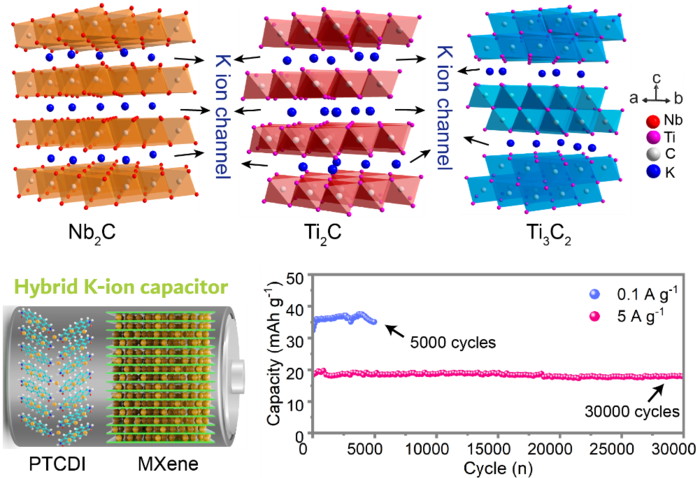 Three different typical MXene electrodes, i.e., Nb2C, Ti2C and Ti3C2, are investigated upon their electrochemical behaviors for aqueous K-ion storage, featuring with pseudocapacitive-dominated behaviors, fast kinetics and durable cycling stability. Image Credit: Nano Research Energy
Three different typical MXene electrodes, i.e., Nb2C, Ti2C and Ti3C2, are investigated upon their electrochemical behaviors for aqueous K-ion storage, featuring with pseudocapacitive-dominated behaviors, fast kinetics and durable cycling stability. Image Credit: Nano Research Energy
A new study performed by the City University of Hong Kong illustrates the excellent performance of a capacitor designed with MXene compounds. MXenes are known as two-dimensional inorganic compounds whose big molecular surface areas for energy storage provide them with storage capacity and ultrahigh conductivity.
The study has been reported in the Nano Research Energy journal on March 21st, 2022.
Supercapacitors have the ability to store enormous energy in a small space and liberate it at a high current. For instance, they could supply power for small devices like wearable electronics. However, despite being made with organic molecules, supercapacitors are prone to catching fire.
The new study examined supercapacitors made with inorganic MXene molecules in the hope of minimizing fire risk. Rather than the more costly lithium, the researchers utilized potassium. The potassium ion or K-ion is considered one of the most commonly utilized electrolytes to enable electrical current to flow in a battery.
We have investigated the aqueous supercapacitors by utilizing the intrinsic safe water-based electrolytes and focused on K-ion storage, which is cheaper and more abundant in earth to benefit for safe and low-cost applications.
Guojin Liang, Study Lead Author and Researcher, Department of Materials Science and Engineering, City University of Hong Kong
MXenes compounds comprise several-atoms-thick layers of transition metals, like metal nitrides, carbides or carbonitrides. They possess the electrical properties of effective electron transport throughout the conductive metal carbide layer, as well as a metal surface that seems great for redox (electron transfer) reactions.
From the different MXenes, this study chose three for comparison of performance.
By horizontally comparing the K-ion storage performance of three representative MXene species, we want to figure out the relationship between the structure and their K-ion storage performance.
Xinliang Li, Study Lead Author, Department of Materials Science and Engineering, Institute of Mechanics, Chinese Academy of Sciences
The three MXene electrodes or so-called electrical conductors - Ti2C, Nb2C, and Ti3C2 - were examined for their electrochemical behaviors. This includes the chemistry of how the K-ions became embedded in the MXene layers and how ions joined to the metal surfaces.
The scientists assessed the supercapacitor's capacity, storage mechanism, cyclic performance and rate performance.
The K-ion capacitor along with the Nb2C MXene exhibited the most excellent performance, consisting of the highest power density (amount discharged) of 2336 W/kg and also an energy density (amount stored) of 24.6 Wh/kg.
Whilst lithium-ion batteries exhibit greater energy density compared to the capacitors, their power density is only in the range of 250 to 340 W/kg. Thus, a K-ion capacitor with MXene can liberate power orders of magnitude faster.
The capacitor with Nb2C MXene retained almost complete capacity (94.6%) following 30,000 cycles of discharging five amperes/g of electricity, in contrast to the approximately 500 cycles a lithium-ion battery is anticipated to survive.
All the MXene materials displayed supercapacitor behaviors, quick kinetics and durable K-ion storage, thereby offering better performance compared to other K-ion host materials. The outcomes stem from the firm structure of MXene as it gets and abandons potassium ions.
It could be ascribed to the intrinsically large interlayer distance for K-ion transport and the superb structural stability of MXene, even subjected to long-term potassiation/depotassiation process.
Guojin Liang, Study Lead Author and Researcher, Department of Materials Science and Engineering, City University of Hong Kong
Although just three MXene electrodes were analyzed, other MXene compounds might have a huge ability to act as aqueous K-ion host electrodes. The scientists hope their outcomes will “draw further attention to other promising MXene electrodes for durable K-ion storage.”
The scientists plan to experiment with MXene additional electrodes to enhance performance for practical applications.
“Regarding the K-ion capacitor, we would like to modify and manipulate the MXene electrode species for higher energy density,” stated professor Chunyi Zhi.
Eventually, the researchers aim to improve K-ion capacitors for wearable electronics and other small power devices, as they are secure, high performing and comparatively affordable.
The study authors include Xinliang Li, Guojin Liang, Yanbo Wang, Shuo Yang, Zhaodong Huang, Qi Yang, Donghong Wang, Binbin Dong, Minshen Zhu, Chunyi Zhi.
This study was financially supported by the General Research Fund (Hong Kong), the City University of Hong Kong, and the Science Technology and Innovation Committee of Shenzhen Municipality.
Journal Reference:
Jiang, L., et al. (2022) Design strategies for low temperature aqueous electrolytes. Nano Research Energy. doi.org/10.26599/NRE.2022.9120003.