Scientists from Zhejiang University and Chongqing University in China have reported the development of novel composite Ag-CeO2 aerogels for photocatalytic CO2 reduction applications. Their findings have appeared in the journal ACS Applied Energy Materials.

Study: Ag–CeO2 Composite Aerogels as Photocatalysts for CO2 Reduction. Image Credit: oxinoxi/Shutterstock.com
Aerogels: Promising Photocatalyst Candidates
Aerogels, which possess the benefits of small particle sizes, large surface area, enhanced porosity, and excellent charge transfer pathways, have been widely explored for several industrial applications. Recently, research has focused on utilizing these materials as photocatalysts.
Modifying aerogels with nanostructured metallic particles can enhance their photocatalytic activity for use as semiconductors in applications such as dye degradation and water splitting. Incorporating these materials induces the surface plasmonic resonance effect. Recent research has demonstrated the development of TiO2-based aerogels, which incorporate metallic species such as silver, gold, platinum, and copper.
These composite aerogels have been studied in liquid media, and typically, they are synthesized by sol-gel methods. Photodepositing metal nanoparticles onto the surface of aerogels have also been explored to produce composites in recent years. Sol-gel methods offer enhanced charge transport properties by ensuring better contact between the composite elements in these aerogels. Additionally, multicomponent aerogels have been evaluated for hydrogen evolution reactions.

The Tauc plots and the appearance images of the photocatalyst. Image Credit: Wu, M et al., ACS Applied Energy Materials
Recent Studies
Photocatalytic metal/metal oxide composite aerogels have been extensively explored in recent studies. There is particular research interest in using them on gas-solid interfaces to prevent potential structural degradation in liquid media. Moreover, using these interfaces for reactions better utilizes the aerogel’s microstructural advantages.
Research has investigated their suitability for the photodegradation of toluene, photocatalytic carbon monoxide oxidation, and photocatalytic reduction of CO2. This last application is especially useful as CO2, which is a greenhouse gas, can be converted into value-added products such as biofuels and industrial chemicals using photocatalysis. 3D monolithic Au-TiO2 aerogels have been used to convert CO2 into methanol in recent studies.
The Study
The authors have evaluated the photocatalytic activity of an Ag-CeO2 composite aerogel on a gas-solid interface for CO2 reduction reactions. In the aerogel, Ag acts as the plasmonic sensitizer, whilst CeO2 acts as the semiconductor. CeO2 was selected by the authors due to the previously reported suitability of this metal oxide as a photocatalyst or cocatalyst.
CeO2 does, however, suffer from low visible light absorption due to wide band gaps. This performance limitation has also been reported in TiO2, another commonly used metal oxide in photocatalytic aerogels. Recent studies have demonstrated the superior surface plasmonic effect of Ag, which has led to its adoption in several plasmon-enhanced reactions. As stated by the authors, the advantages of both materials could be beneficial for photocatalytic reactions.
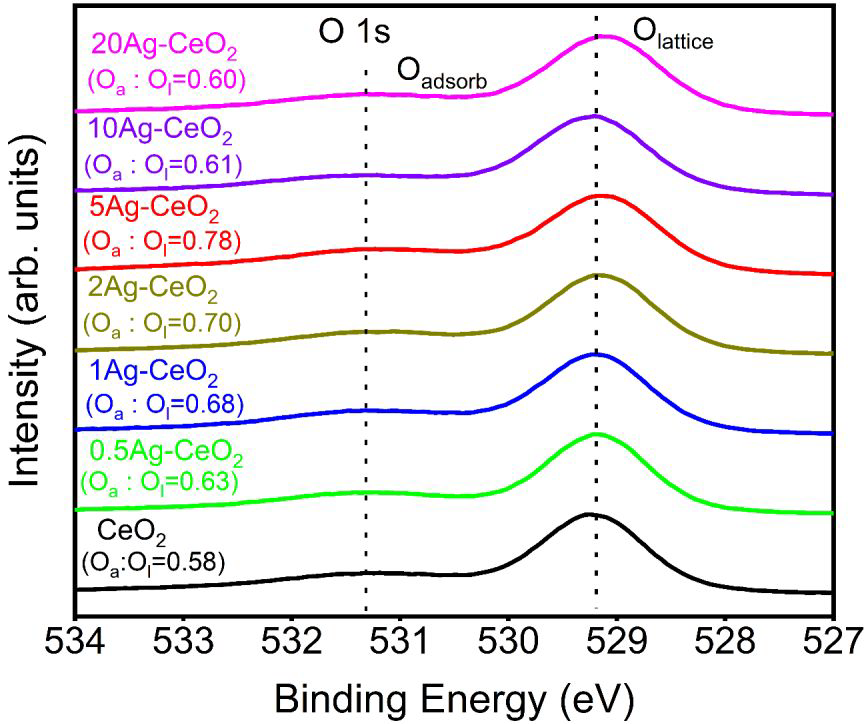
Detailed scan of O 1s core level of CeO2 and Ag-CeO2 aerogels. Image Credit: Wu, M et al., ACS Applied Energy Materials
The prepared aerogel possesses abundant interfaces between the semiconducting CeO2 and plasmonic sensitizing Ag, rich oxygen vacancies, and interconnected charge transfer pathways. These properties potentially give the material enhanced gas-phase photocatalytic CO2 reduction performance.
The composite aerogels were produced by a modified dispersive sol-gel method and supercritical drying. No pre-synthesized nanoparticles were used in their synthesis. This modified synthesis method produced materials with a highly porous architecture with stacked nanoparticles. A final calcination step was used to produce the photocatalytic aerogels.
Aerogel Performance
Photocatalytic activity studies revealed that the composite Ag-CeO2 aerogel performed sufficiently for CO2 reduction, with high yields of CH4 produced in reactions. Furthermore, the aerogel possesses high selectivity under visible and full-spectrum light radiation. This enhanced activity was mainly due to the action of Ag, which generated a surface plasmonic resonance effect. The inclusion of Ag increased the aerogel’s separation efficiency and charge carrier generation rate.
Further studies demonstrated that Ag inclusion also has the effect of trapping defect-related electrons in visible light. Due to this material characteristic, the composite aerogel possesses high CO2 reduction activity when irradiated by broadband wavelengths.
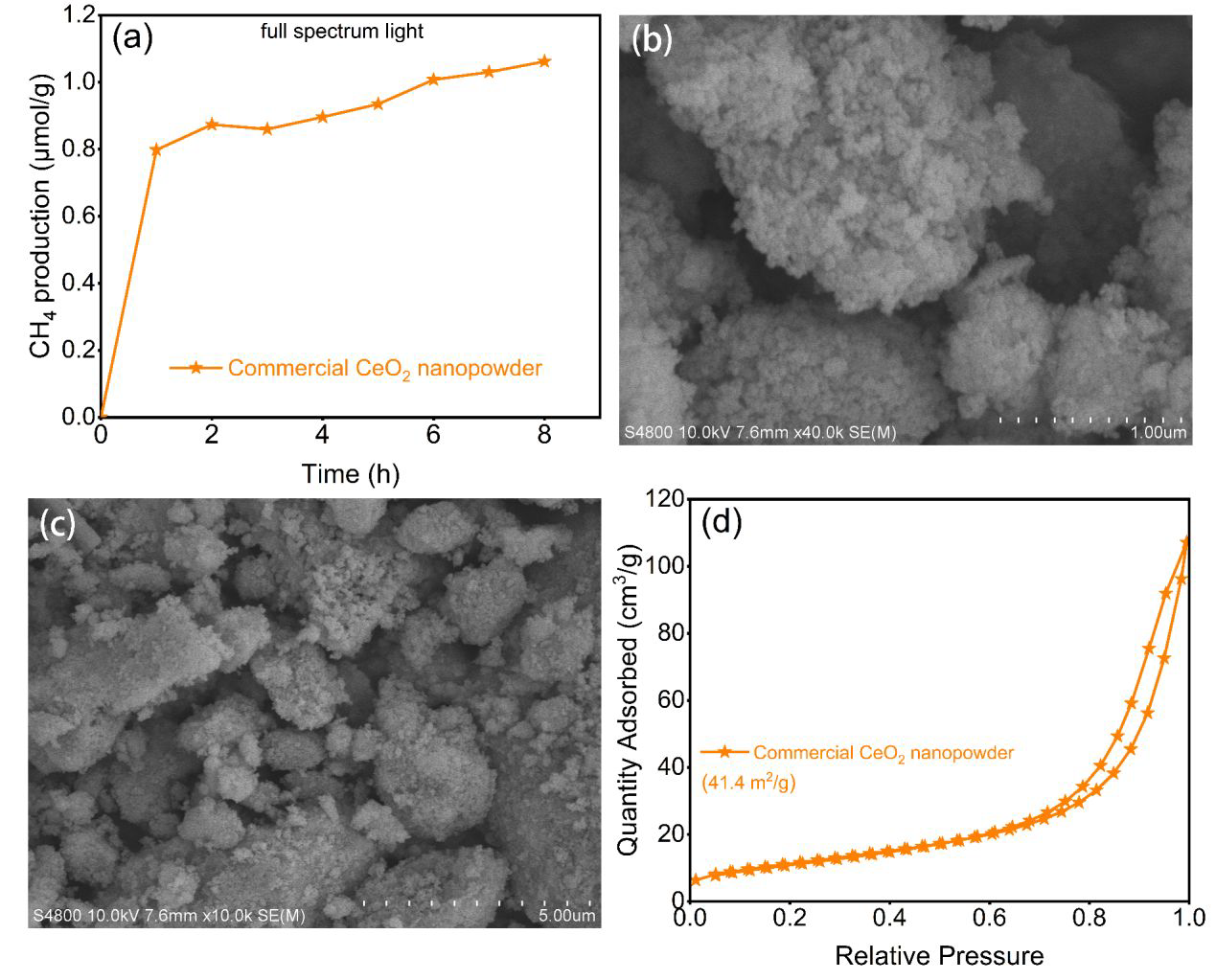
(a) CH4 production on commercial CeO2 nanopowder under the full-spectrum light, (b) and (c) are the SEM images of the commercial CeO2 nanopowder, and (d) is the corresponding N2 adsorption/desorption isothermal curves. Image Credit: Wu, M et al., ACS Applied Energy Materials
In Summary
The authors have demonstrated the development of a novel metal/metal oxide composite aerogel for CO2 reduction reactions. This material displays enhanced photocatalytic activity, charge carrier transport, and high selectivity across a broad range of light radiation. The authors have stated that this aerogel can be considered a “clean” catalyst, with organic residue decomposition producing no artificial results.
Manufacturing materials for CO2 reduction applications is a crucial research area in materials science and engineering at the moment due to the effects of climate change and the need to produce sustainable, value-added products from CO2 to realize the aims of the circular economy. The research has provided a significant contribution to the field of aerogel-based photocatalysts.
Further Reading
Wu, M et al. (2022) Ag–CeO2 Composite Aerogels as Photocatalysts for CO2 Reduction ACS Applied Energy Materials [online] pubs.acs.org. Available at: https://pubs.acs.org/doi/10.1021/acsaem.2c00852
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.