A team of researchers recently published a paper in the journal Materials that have demonstrated the feasibility of using boron-doped diamond (BDD) thin film electrodes to detect trace lead (Pb2+) in drinking water.

Study: Fabrication of Boron-Doped Diamond Film Electrode for Detecting Trace Lead Content in Drinking Water. Image Credit: Kim Britten/Shutterstock.com
Background
The discharge of heavy metal pollutants from different sources, such as agricultural and industrial sources, into natural water bodies has significantly contributed to water pollution and severely deteriorated the quality of water, specifically drinking water.
Among these pollutants, Pb2+ is the most harmful heavy metal pollutant as it is highly toxic, difficult to degrade, and can easily accumulate in the human body. In drinking water, excessive Pb2+ content can adversely affect the human body by irreversibly damaging the nervous system, reproductive system, and internal organs.
Growing concerns about Pb2+ pollutants in drinking water have increased the importance of developing reliable analytical techniques to monitor the water quality to ensure the safety of drinking water.
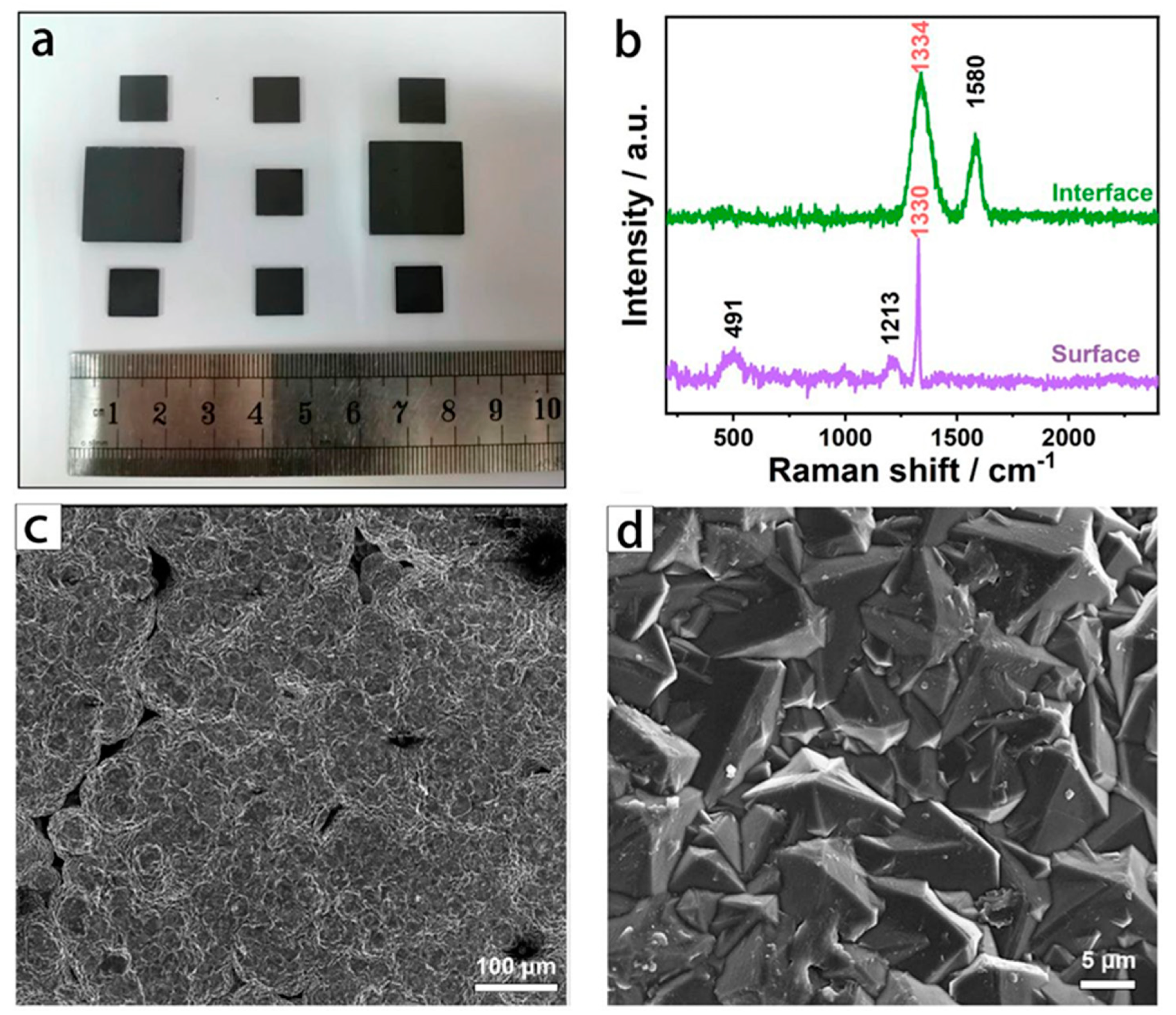
Structure of BDD electrode. (a) Digital photographs; (b) Raman spectra; (c,d) SEM images at two magnifications. Image Credit: Wu, L et al., Materials
Atomic absorption spectrometry, flow injection analysis, and liquid chromatography is typically used to detect trace Pb2+ in water. However, these analytical methods are expensive and extremely complex, which limited their application for monitoring water quality.
Electrochemical analysis technologies, including differential pulse voltammetry and square wave stripping voltammetry, are suitable for Pb2+ detection owing to their fast signal response and high sensitivity.
In an electrochemical detector, the detection electrode is the primary component that determines the analytical capability of the detector. Currently, metal film electrodes, such as gold and antimony film electrodes, and mercury film electrodes are commonly used as detection electrodes.
However, these electrodes have a short life and poor stability, which necessitated the development of new electrode materials. BDD, a new carbon material, can be used as a detection electrode as it possesses the functional performance of semiconductors and good chemical and physical properties of diamonds.
Specifically, good electrochemical properties such as high potential window, low background current, exceptional chemical stability, and high hardness of BDDs make them suitable for application in advanced oxidation technologies. Thus, BDD-based electrodes for heavy metal analysis can be used to achieve high reliability and stability in water quality monitoring.
However, sufficient studies have not been performed using BDD film electrodes to detect Pb2+ in drinking water.
The Study
In this study, researchers synthesized a BDD film electrode to detect trace Pb2+ in water. Later, researchers systematically investigated the electrochemical and microstructural behavior and the Pb2+ detection ability of the synthesized BDD film electrodes to assess the feasibility of these electrodes to monitor heavy metals.
A hot-filament chemical vapor deposition (HFCVD) system was employed to prepare the BDD film electrode. A porous titanium metal slice was utilized as the BDD electrode substrate. Initially, the substrate is cleaned and placed in a diamond seed solution containing nanodiamond and ethanol for 10 min for seeding treatment. Then, the substrate was dried and transferred to an HFCVD chamber for deposition.
Subsequently, diboron trioxide dissolved in ethanol was used as a doping boron source and brought into the HFCVD chamber using hydrogen gas. Hydrogen and methane were used as an etching gas and a carbon source, respectively, and the hydrogen:methane ratio was set as 10:1000 sccm for 30 min in the nucleation stage. In the growth stage, the hydrogen gas flow was adjusted to ethanol + hydrogen + diboron trioxide:hydrogen = 25:50:1000 sccm for 7.5 h.
The thickness of the layer was influenced by the product of the deposition time and deposition rate. The carbon/hydrogen (C/H) ratio, boron/carbon (B/C) ratio, deposition pressure, and deposition temperature during the deposition process were 2.4%, 6000 ppm, three KPa, and 700 oC, respectively.
A scanning electron microscope (SEM) was used to evaluate the electrode micromorphology, while an electrochemical workstation was employed to perform the electrochemical test. A Raman confocal microscope was employed to detect the binding structure of the electrode.
During electrochemical measurements, a 10 × 10 mm2 BDD film was utilized as the working electrode, while a saturated glyceryl electrode and a platinum sheet were used as the reference electrode and counter electrode, respectively.
Researchers also performed cyclic voltammetry (CV) test to evaluate the detection electrode potential window, reaction kinetics, and background current, and electrochemical impedance spectroscopy (EIS) to observe the electrochemical reaction process in the 10−2–105 Hz frequency range. Additionally, square wave dissolution voltammetry (SWASV) was performed to detect Pb2+ in the water.
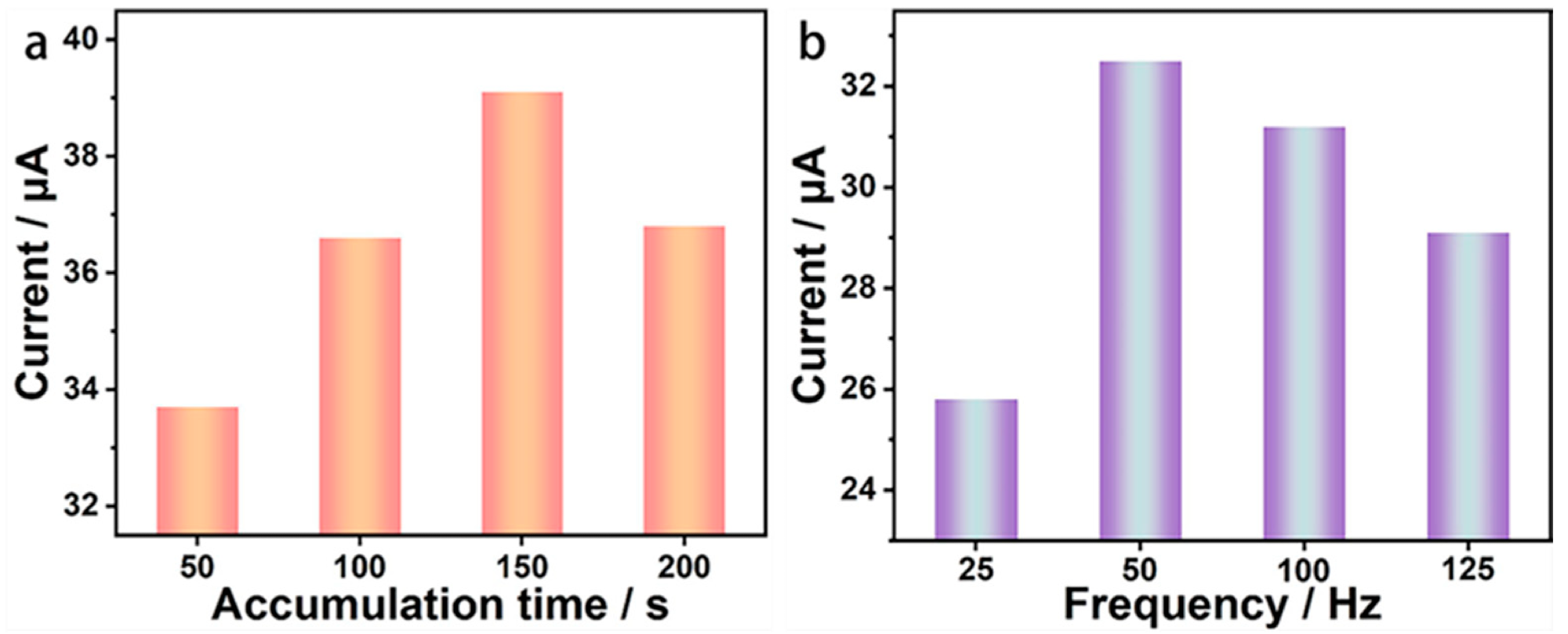
Effect of enrichment time and scan frequency on the signal response of the BDD electrode. (a) Dissolved peak current values of BDD electrode for Pb2+ at four enrichment times; (b) Dissolved peak current values of BDD electrode for Pb2+ at four scanning frequencies. Image Credit: Wu, L et al., Materials
Observations
A BDD film electrode was prepared successfully using the HFCVD process. No macroscopic defects, such as cracking and flaking, were observed on the electrode surface. Raman spectra confirmed successful boron doping into the diamond lattice of the BDD film. The diamond grains arranged on the BDD electrode surface featured a high phase quality.
The BDD film primarily contained boron and the diamond phase, which was suitable for broadening the potential window and decreasing the electrode noise. Additionally, the amorphous carbon phase at the BDD film interface was effective as a conductive medium to facilitate electrical signal transmission.
The BDD electrode displayed a wide potential window of 2.2 V, which effectively facilitated the Pb2+ dissolution analysis by the electrode. Moreover, the electrode possessed a large electrochemical active area of 4.38 cm2 and displayed an extremely low background current, which improved the signal ratio and sensitivity and suppressed the electrode noise of the BDD electrode.
Moreover, the low charge transfer resistance of 6.54 ω of the electrode ensured that the electrochemical reaction can progress smoothly, resulting in an improvement in signal response. These factors collectively accelerated the Pb2+ stripping reaction process.
During the trace Pb2+ detection test, the scanning frequency and enrichment time were optimized to obtain a better signal response. A scanning frequency of 50 Hz and an enrichment time of 150 s were selected as optimized dissolution parameters for sensitivity detection and anti-interference ability experiments.
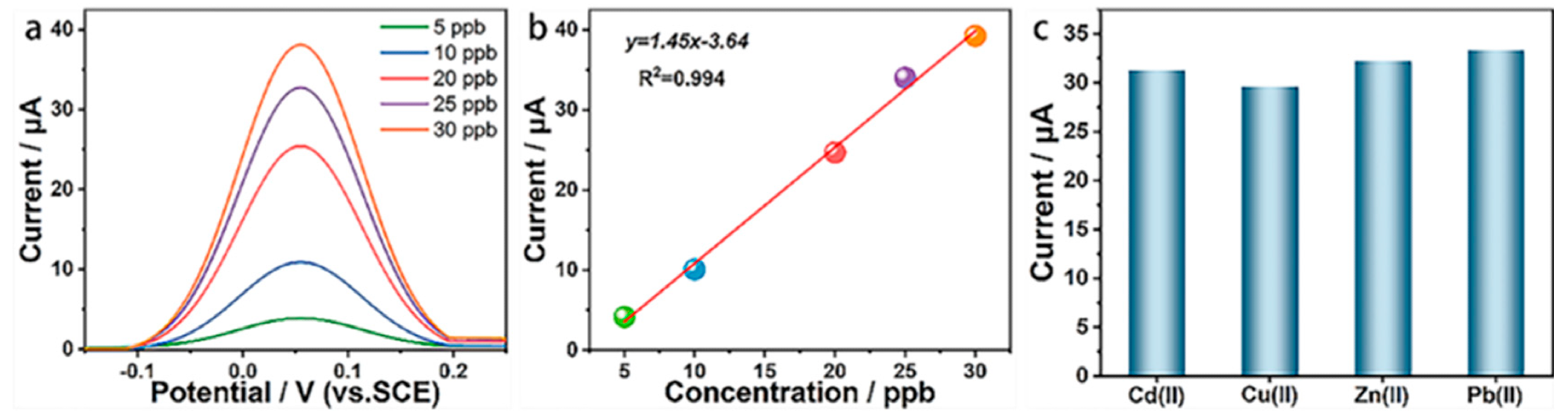
Detection performance of BDD for Pb2+. (a) Dissolution curves of five Pb2+ concentrations; (b) Linear fitting curve between Pb2+ concentration and dissolution peak current; (c) Dissolution peak current before and after addition of interfering ions. Image Credit: Wu, L et al., Materials
The synthesized electrode demonstrated an excellent Pb2+ selectivity of 1.45 µA L µg−1 cm−2 in the Pb2+ concentration range of 5-30 ppb, a low Pb2+ detection limit of 2.62 ppb, and good anti-interference ability. Overall, the BDD electrode demonstrated an improved detection performance and good electrochemical behavior.
To summarize, the findings of this study demonstrated the feasibility of using BDD film electrodes to achieve reliable and stable monitoring of trace Pb2+ in drinking water.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Wu, L., Liu, X., Yu, X., et al. Fabrication of Boron-Doped Diamond Film Electrode for Detecting Trace Lead Content in Drinking Water. Materials, 2022. https://www.mdpi.com/1996-1944/15/17/6013