 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Oct 7 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Oct 7 2022In an article recently published in the journal Advanced Sustainable Systems, researchers discussed the challenges and opportunities around the use of black phosphorus for rechargeable batteries and electrocatalysis.

Study: Opportunities and Challenges of Black Phosphorus for Electrocatalysis and Rechargeable Batteries. Image Credit: Luckymane/Shutterstock.com
Background
The most stable phosphorus allotrope is called black phosphorus (BP), and it is a newly discovered 2D layered substance. Theoretically, BP has significant electrochemical ion storage capacities and is the most thermodynamically stable phosphorus allotrope. The 2D material family is enriched by the unique features of phosphorene, a monolayer that was exfoliated from the bulk BP crystals. Black phosphorus quantum dots (BP-QDs) are being developed as a multifunctional nanomaterial for a variety of environmental and sustainable energy applications. Despite the clear benefits of BP, some of its inherent qualities are inadequate for a few particular applications.
The creation of BP-based heterostructures can greatly enhance the characteristics and hence expand the application of BP-based materials as a successful solution to these problems. Potential electrocatalysts for several reactions, including the oxygen evolution reaction (OER), oxygen reduction reaction (ORR), hydrogen evolution reaction (HER), nitrogen reduction reaction (NRR), etc., have been investigated using BP.
Increased active sites, surface reconstruction, and chosen adsorption paths for electrocatalysis can all be accomplished by physically building BP-based heterostructures. A promising electrode material for rechargeable batteries is BP because of its extremely high theoretical capacity. BP-derived hetero-structure electrodes have been researched for improving conductivity, limiting large-volume swelling, and inducing the stable solid-electrolyte interphase to further enhance battery performance.
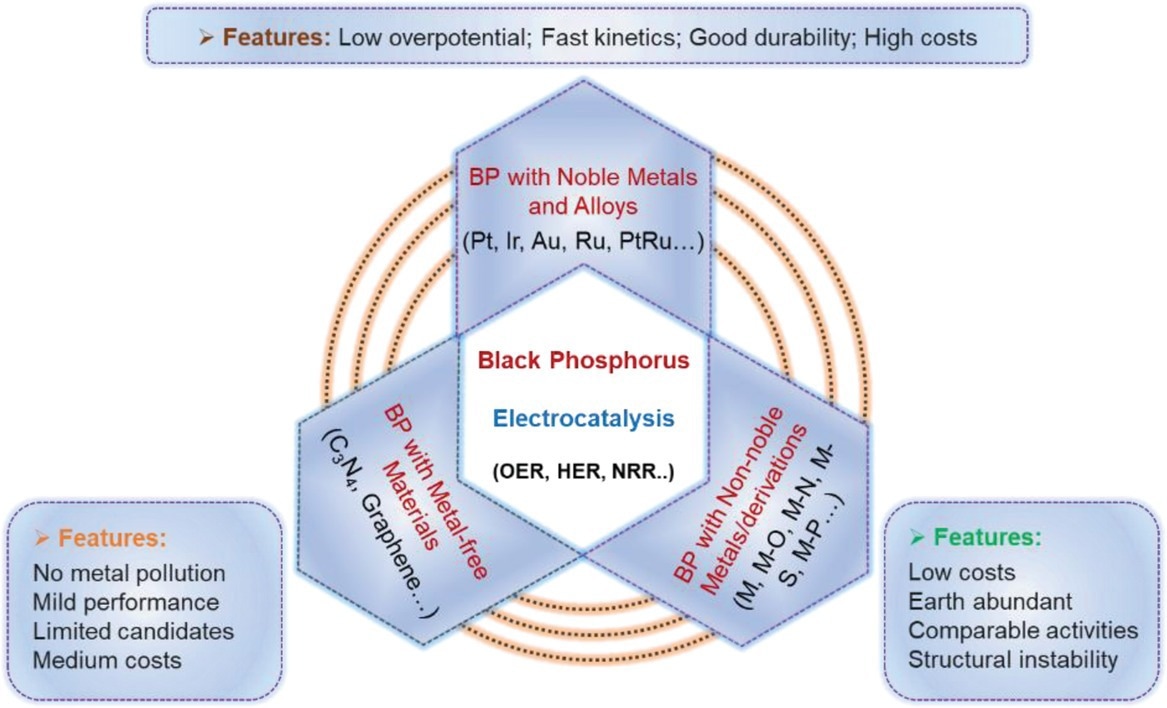
Schematical illustration of three primary types of BP-derived catalysts and their main features for electrocatalysis. Image Credit: Mei, J et al., Advanced Sustainable Systems
About the Study
In this study, the authors provided a brief overview of the most recent developments in electrocatalysis, including the oxygen evolution reaction (OER), hydrogen evolution reaction (HER), and nitrogen reduction reaction (NRR), as well as in rechargeable batteries, including sodium-ion batteries (SIBs), lithium-ion batteries (LIBs), and potassium-ion batteries (PIBs). This was followed by a critical assessment of the current opportunities and challenges. It was anticipated that this category of emerging materials would lead to fresh ideas for potential responses to the current energy issue and would motivate scientists to create further unique materials for upcoming multifunctional uses.
The team examined the uses of BP in cutting-edge electrocatalysis and rechargeable batteries. A summary of current developments in the regulation of BP and BP-derived hybrids in electrocatalysis and rechargeable batteries, including LIBs, PIBs, SIBs, and Li-S batteries, was provided.
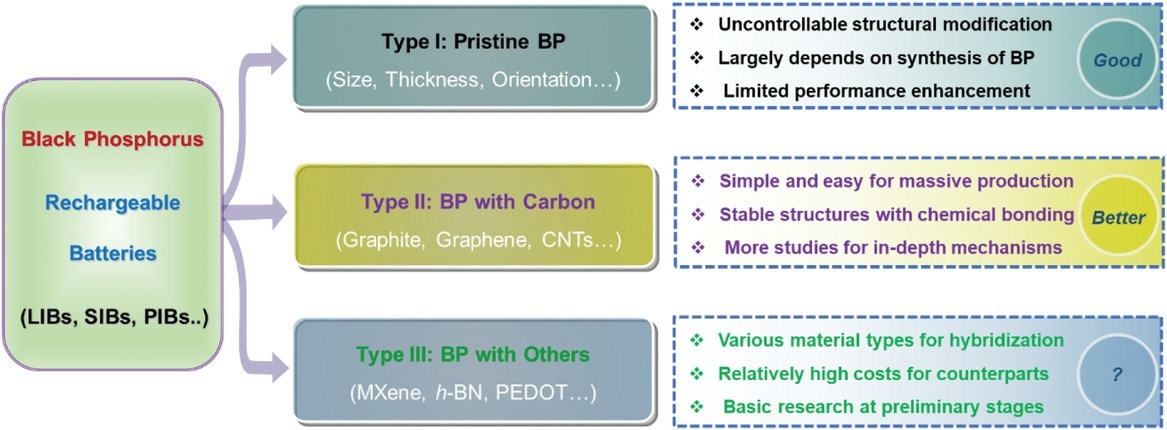
Schematic illustration of BP and BP-derived composites and their main features for rechargeable batteries. Image Credit: Mei, J et al., Advanced Sustainable Systems
Observations
An electrical conductivity of 88.9 Ω, a discharge capacity of 1088 mA h g-1 at 0.1 A g-1, cycling stability with 757.3 mA h g-1 at 0.5 A g-1 over 650 discharge/charge cycles, and rate performance with 552 mA h g-1 at a high rate of 2.5 A g-1 were reported for the BP/carbon nanotubes (CNTs) electrode for LIBs. According to one study, Ni2P/BP heterostructures boosted electrical conductivity up to 6.25 x 104 S m-1, which in turn increased the charge carrier concentration to 1.37 x 1020 cm-3 and enabled steady HER cycling that lasted for more than 3000 cycles.
A 2505 mA h g-1 first discharge capacity at 4 GPa and 400 °C was recorded for the BP made from white phosphorus, whereas a 2649 mA h g-1 initial discharge capacity at 4.5 GPa and 800 °C was reported for the BP made from red phosphorus. Additionally, BP faced considerable challenges concerning large-scale production and secure storage, and the current cost was very expensive. Because of the limitations of synthetic methods, it was exceedingly difficult to accurately control the quality and assembly of BP-based heterostructures. To promote BP stability, surface coating, changes, or doping were proven to be successful.
The majority of current research on BP focused on LIBs and SIBs, in which the electrochemical ion storage methods were comparable for both ions, except for the performance difference caused by the different ionic radii between Na+ and Li+. The use of conductive carbon materials in a hybrid with BP was a successful strategy for dealing with the problems caused by structural changes brought on by repeated lithiation/sodiation processes.
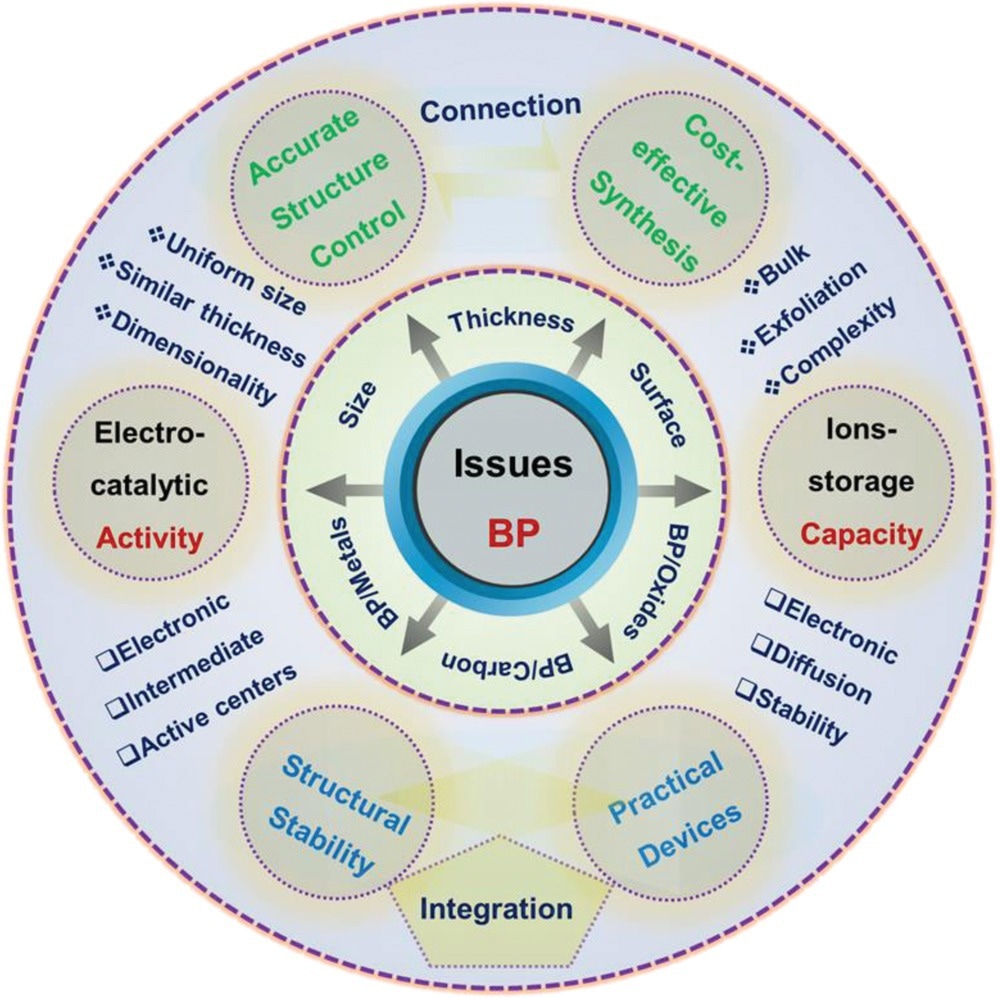
Schematic illustration of key issues for BP and its derivations for electrocatalysis and rechargeable batteries. Image Credit: Mei, J et al., Advanced Sustainable Systems
Conclusions
In conclusion, this study presented in-depth research on the potential of BP and BP-derived composites as efficient catalysts for a variety of electrocatalytic reactions, as well as electrode materials for a variety of rechargeable batteries. Due to the varied quality and surface and chemical states, the BP produced using various synthetic methods had a variety of electrochemical characteristics.
The team determined that despite significantly improved performance for BP-based materials in batteries and electrocatalysis, there is still a huge gap between lab-scale testing and industrial production, which is a result of the pristine BP's environmental sensitivity and the small volume production technologies for nanomaterials.
The authors mentioned that more work is urgently needed to improve the structural stability and catalytic capabilities of materials based on BP. They stated that it is necessary to use a combination of theoretical calculations and experimental research to uncover intricate reaction mechanisms.
References
Mei, J., Liao, T., Sun, Z., Opportunities and Challenges of Black Phosphorus for Electrocatalysis and Rechargeable Batteries. Advanced Sustainable Systems, (2022). https://onlinelibrary.wiley.com/doi/10.1002/adsu.202200301.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.