Expanding the pores of metal-organic frameworks (MOFs) by chemical etching might enhance the MOFs’ performance in several types of applications, such as catalysts and fuel cells. The novel technique was created by researchers from East China Normal University and Nagoya University in Japan, collaborating with partners in Australia, China, and other countries. Their findings were published in the Journal of the American Chemical Society.
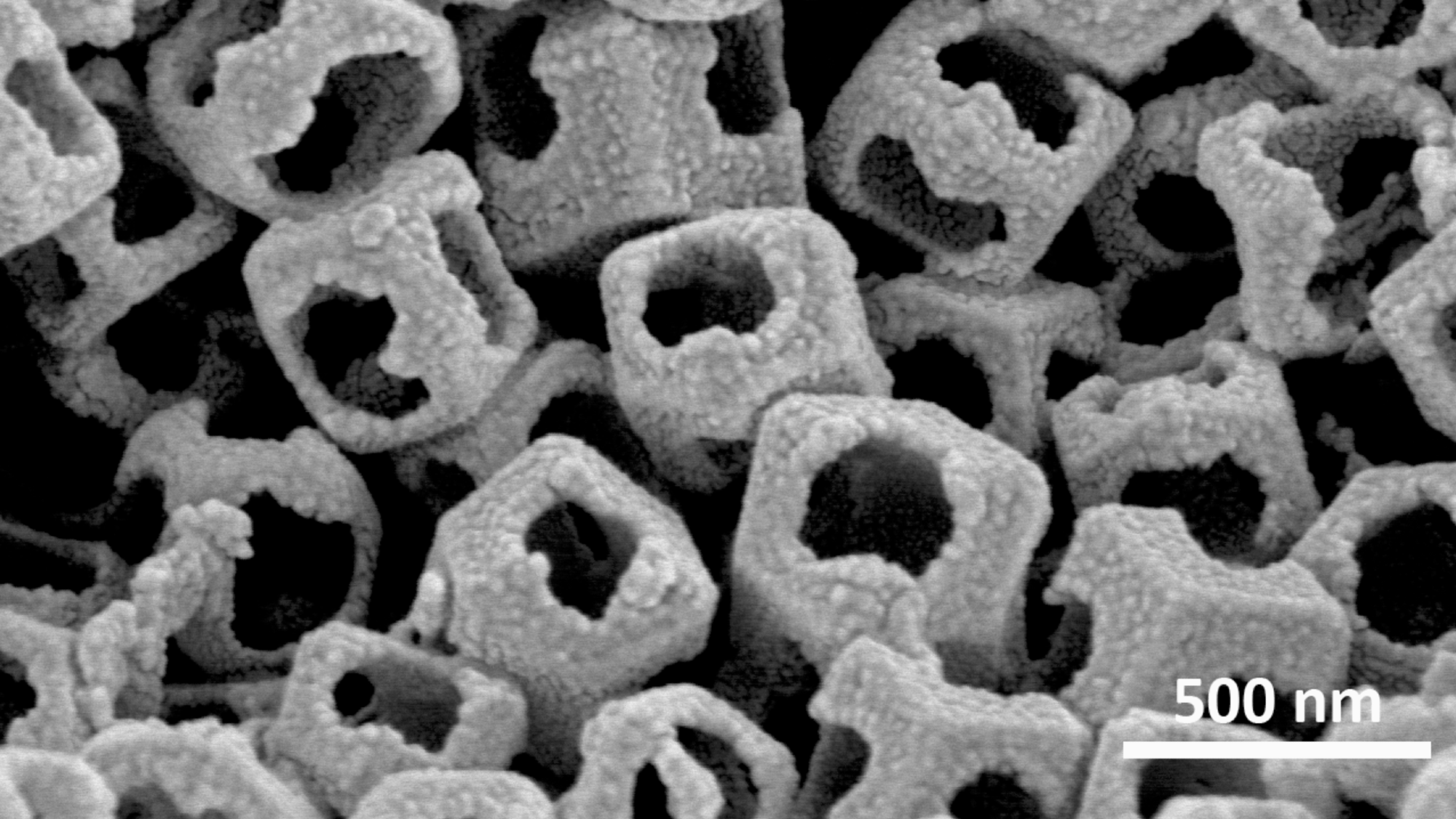 Structure of the etched open-pore MOF. Image Credit: Wei Xia
Structure of the etched open-pore MOF. Image Credit: Wei Xia
Metal-organic frameworks (MOFs) are porous materials consisting of metal ions or clusters interconnected by organic linker groups based on carbon. Different MOFs can be created by varying the metallic and organic components, which can be used for several purposes, such as chemical separation, gas storage, and catalysis.
It is evident that certain MOFs have the ability to catalyze the chemical processes seen in fuel cells, which are being investigated as the foundation for renewable energy sources. Fuel cells have the potential to be extremely important in the shift to a low- or zero-emission economy to fight climate change because they require no fossil fuels.
However, there has been a problem in using MOFs, because the catalyst layer is too thick and their pore structure is insufficiently open to allow the necessary transfer of chemicals. This exacerbates the sluggish mass-transport properties of the catalyst layer, and limits the application of MOFs in many renewable energy systems, particularly for proton-exchange membrane fuel cell (PEMFC) applications. So, a growing interest has been in constructing hollow MOFs with open pore structures to boost reactant penetration and shorten mass diffusion paths.
Yusuke Yamauchi, Professor, Nagoya University
Yamauchi added, “This makes it possible for us to elaborate unprecedented morphologies and hollow structures with open pore structures in a single MOF nanoparticle as a precursor for PEMFC catalysts, unlocking the potential of advanced materials for PEMFC applications.”
Another obstacle to the utilization of current MOFs has been their chemical instability.
To etch a more open structure throughout a MOF, the researchers employed chemical combinations. Following an initial etching cycle, the MOF’s interior grew more porous, allowing iron ions—essential for catalysis—to be loaded into the structure.
This MOF’s open structure contains individual iron ions, each of which is catalytically active. The final catalysts, known as OP-Fe-NC, were created by calcining the final MOF under an inert environment.
Preliminary models indicate that this structure will significantly boost the transport of oxygen through the material, increasing its activity and stability.
Our promising results highlight the potential of OP-Fe-NC as an effective electrocatalyst for various energy storage and conversion devices. For this work using OP-Fe-NC as a cathode catalyst delivered extraordinary Oxygen Reduction Reaction (ORR) activity and excellent stability in acidic media, which is even better than the commercial Platinum/Carbon catalyst. In the fuel cell, OP-Fe-NC showed the high current density which was close to the US Department of Energy (DOE) 2025 target.
Wei Xia, East China Normal University
Xia stated, “This work provides a new approach for designing and optimizing high-efficiency catalysts for ORR by simultaneously increasing the intrinsic catalytic activities of the active sites and effectively utilizing the active sites in the catalyst layer.”
After proving the method’s theoretical viability, the researchers now want to investigate how additional chemical modifications could enhance the strategy and yield materials appropriate for various real-world scenarios.
“We intend to bridge the gap between experimental work and practical applications, hopefully making a real contribution to the drive towards sustainable energy solutions,” Yamauchi concluded.
Journal Reference:
Li, J., et. al. (2024) Selective Etching of Metal–Organic Frameworks for Open Porous Structures: Mass-Efficient Catalysts with Enhanced Oxygen Reduction Reaction for Fuel Cells. Journal of the American Chemical Society. doi:10.1021/jacs.3c05544.
Article Revisions
- Mar 12 2024 - Edited: Nagoya University is in Japan, not China