When discussing the subject of Analytical Instrument Qualification (AIQ), the first thought may be regulated industries, such as food or pharmaceuticals.

Image Credit: Metrohm AG
Typical questions that arise include, why is it necessary to qualify analytical instruments in such environments? Why do titrando or OMNIS systems need such a service?
Here, the safety of consumers is of utmost importance. Medicines that may be considered a health hazard for patients or do not generate the therapeutic effect as intended are unwanted and costly.

Image Credit: Metrohm AG
This means that a series of steps are required to safeguard the manufacturing process and avoid any fatal implications. By qualifying the analytical instruments being used, we can check that active ingredients and pharmaceutical end-products are produced in a safe environment.
Additionally, procedures that verify the accuracy and repeatability of instruments are crucial. Metrohm qualification procedures supply this documentation, fully traceable evidence, which is also necessary for audits and inspections by regulatory bodies.
When Auditors Come Knocking
In case an auditor discovers any breaches of the United States Food and Drug Administration (FDA) guidelines, this will be transmitted via a warning letter or an inspectoral observation.
When evaluating previous pharmaceutical warning letters, it is clear to see the FDA is primarily concerned with issues related to qualification and data integrity.
Typical violations auditors may observe include the use of an unqualified system or utilization of an instrument beyond the calibration range for which it was initially qualified. This demonstrates the fact that the qualification of analytical instruments in regulated environments was brushed aside.

Image Credit: Metrohm AG
Metrohm Compliance Services can help validate the entire data traceability of qualification activities, simplifying preparation for an audit preparation while maintaining a sustained state of being inspection ready for any laboratory.
According to the main regulatory bodies, instruments in regulated environments must be periodically qualified. The United States Pharmacopeia (USP), the leading pharmacopeia, has a general chapter exclusively assigned to Analytical Instrument Qualification (AIQ), USP <1058>.
Consequently, it has a significant impact globally, meaning laboratories are subject to regulatory requirements either directly or indirectly. This is why Metrohm Compliance Services is established in this key chapter.
What is Analytical Instrument Qualification (AIQ) Exactly?
As stated in USP <1058>, it is “the collection of documented evidence that an instrument performs suitably for its intended purpose.” This signifies that AIQ is the basis for produced good-quality data with the requisite data integrity.
By utilizing qualified instruments, confidence is gained in the viability of data generated and that instruments meet the specifications of regulatory standards.
AIQ is anecessary process over the whole lifetime of the instrument. With the formal writing of User Requirement Specifications (URS), where the lab’s requirements for a particular instrument are documented, it is clear to see that AIQ begins prior to the instrument purchase.
For example, it is equally important to document the laboratory requirements and its intended to use a fully equipped Metrohm Dual IC system as well as for a single Metrohm pH meter.
After classifying the intended use and the assessment of the right technology, performing a Risk Assessment (RA) is necessary in order to establish the qualification strategy to validate the ‘fitness for purpose’ of the analytical instrument under purchase.
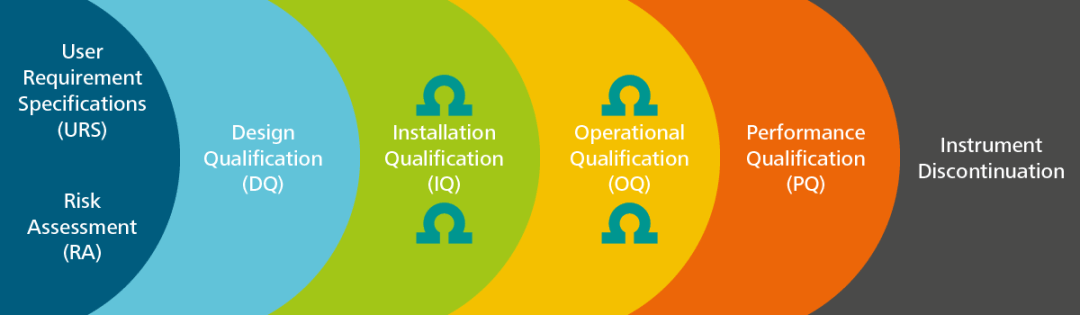
Image Credit: Metrohm AG
The outcome of the Risk Assessment establishes to what extent the subsequent qualification stages are dependent. The following activities are arranged into four phases: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), the so-called ‘4 Q’s.’
Where DQ is the documented verification that the instrument specifications comply with the laboratory requirements, the IQ offers the proof that correct install of the equipment has carried out.
In the OQ phase, it is determined that the system functions properly in the selected environment as per manufacturer specifications, while the PQ signifies that the instrument performs consistently according to pre-defined specifications.
During the instrument lifetime, significant repairs may be necessary, it might be subject to critical updates/upgrades, or it might even be relocated to another lab. In each of these cases, the original URS should be re-evaluated and adjusted as necessary.
Thus, the URS is a living document that can and must be updated when called for. It will then be defined, based on a risk assessment analysis, what qualification steps should be repeated after the requisite changes (IQ, OQ, PQ).
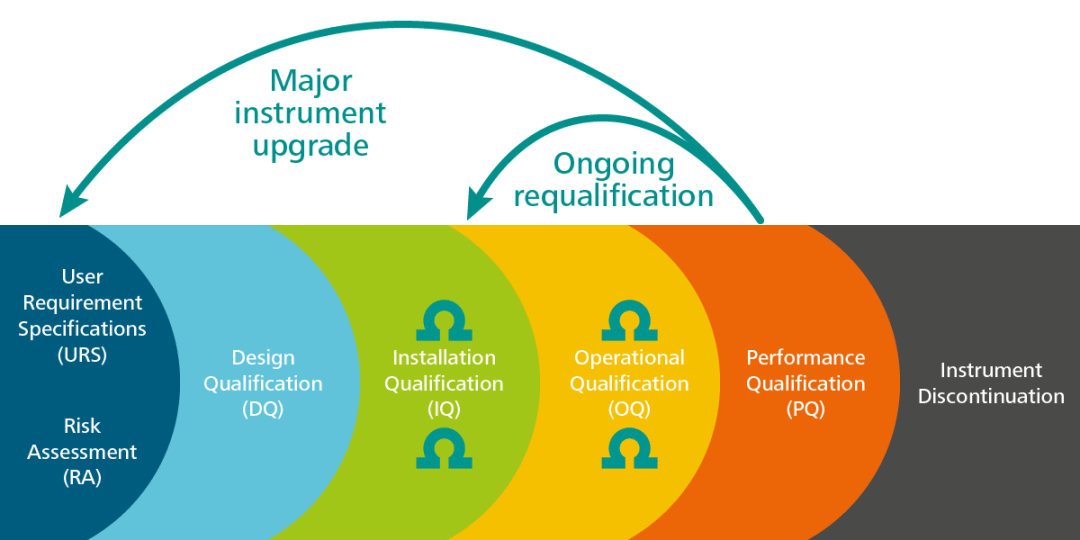
Image Credit: Metrohm AG
Eventually, the life of an instrument comes to an end, which means it must be retired. This last step of the AIQ is generally considered to be the ‘forgotten child’ of validation activities.
To illustrate this clearly, compare this to making a new consumer electronic purchase, such as a PC. The situation is relative to the purchase of a new analytical system.
Ensuring proper training for proper usage and making sure it’s functioning correctly is much easier with newer equipment: it is common to forget or ignore the old system.
Therefore, another critical part of the validation process is the decommissioning of an instrument, one that must also be documented appropriately. For the older system, a final system qualification could be compulsory if required and all data has to be extracted and stored in a safe location.
It is vitally important to make sure that the data is available from this location (data migration) for several years, predicated on the retention procedures in place.
Support When and Where You Need It
While users have responsibilities for the instrument qualification (USP <1058>), help is on hand for certain qualification activities. Metrohm supports users during the course of the lifetime of an investment, from offering advice at the initial purchase process to the first installation and qualification.

Image Credit: Metrohm AG
Additionally, Metrohm’s IQ/OQ documentation supplies the necessary documentation in close compliance with the current regulations.
To ensure a Metrohm device stays in a qualified state and guarantees the accuracy and precision of a system over its lifetime, the company offers requalification services at planned intervals as stated in user requirements.
One of the benefits on offer with Metrohm as the manufacturer of analytical instruments is the company possesses the experience necessary for performing IQ/OQ procedures.
Most crucially, Metrohm’s service engineers carry all calibrated and certified reference instruments that are necessary for the qualification. To maintain the quality of Metrohm Service, service engineers consistently attend compulsory re-training in accordance with a global standardized program.
Buying Metrohm equipment may be the first step to success but maintaining it in a qualified state is crucial! Contact a local Metrohm dealer for more details about which qualification phases can be fully handled by Metrohm.

This information has been sourced, reviewed and adapted from materials provided by Metrohm AG.
For more information on this source, please visit Metrohm AG.