Feb 21 2001

Image Credit: p_i_r_a_n_y_a/Shutterstock.com
Article updated on 12/02/2020 by Jo Finchen-Parsons
Severe local attack, such as pitting, (see figure 1) and crevice corrosion, can be a significant problem in stainless steels and other alloys that depend on self-healing, which can only occur when oxygen is available, adherent, and relatively defect-free oxide films for their resistance to corrosion.
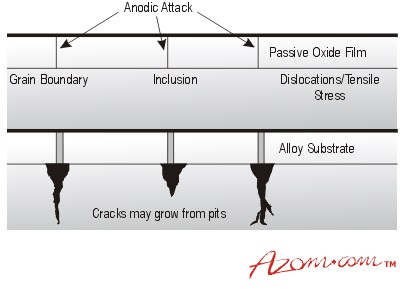
Figure 1. Pitting attack (a) possible defect site and (b) growth from defects.
Pitting
Pitting results from the local breakdown of the barrier film, which allows an anodic reaction to begin at point sites on the exposed metal. The cause of the point site may be due to local mechanical damage, chemical breakdown of the surface film, oxygen depletion beneath debris particles on the surface or chemical (galvanic effect) differences between second-phase particles or inclusions and the metal matrix.
Pitting is quite often self-accelerating due to local rises in acidity in the pits. This process is usually associated with the presence of certain ions, such as chloride and sulfide, in the corrosive environment, both of which can contribute to film breakdown and prevent film repair.
Stainless Steel Design
In stainless steels, a minimum of 12% chromium is required in solid solution in the matrix to provide passivation by protective oxide film formation. Nickel (Ni) additions, which normally account for 6–10% of steels, as well as molybdenum (Mo) and nitrogen (N) are also used to improve general corrosion and pitting resistance. Each of these elements also help to control matrix structure, such as when austenitic or duplex grades or being produced. In these steels, carbon content is kept as low as possible during alloy production. Subsequent processing, welding, and heat treatment variables are carefully controlled to avoid the formation of chromium (Cr)-rich carbide precipitates and other damaging second-phases, all of which can reduce toughness and also cause severe pitting and intergranular attack due to micro-galvanic effects.
A row of pits can also form along deep scratches as the oxide film will not be impervious and the underlying metal will contain additional internal stress. Larger pits can form at dross and sand inclusions, highlighting the damaging effect of inadequate casting cleanliness. Casting defects, such as shrinkage pores and inclusions, can also result in a severe local attack.
To minimize pitting, stainless steels are solution-treated and quenched to dissolve second-phase precipitates and ultimately prevent their reformation upon cooling. Due to the presence of chloride ions, conventional austenitic and duplex grades are prone to both pitting and crevice corrosion in seawaters; however, their resistance can be improved by additions of up to 6% Mo and 0.2–0.5% N.
Predicting Pitting Resistance
The relative behavior of various grades can be compared through the use of an empirical relationship for Pitting Resistance Equivalent (PREN), which is based upon laboratory corrosion tests in chloride-containing solutions:
PREN = %Cr + 3.3%Mo X%N
In this equation, X = 16 for duplex and X = 30 for austenitic steels. Note that a higher the PREN value represents better pitting resistance.
Hence, super-duplex and super-austenitic grades give PREN values of 40–50, lean alloy duplex gives values of 27–30 and 18/8 (type 304) austenitic gives a value of about 20. For duplex structures, alloys are designed to produce ferrite and austenite phases with the same pitting resistance to avoid the preferential attack of either phase.
Research into pitting and the growth of corrosion fatigue and stress corrosion cracks, which are believed to originate at pits, can now be aided by the use of special techniques. These techniques allow for real-time mapping of local corrosion activity and the determination of localized corrosion rates. Such information is essential in the accurate prediction of safe working life.