Offering a new concept for infrared spectroscopy, the mid-level IRXross FTIR spectrophotometer achieves upper-end performance, including high-level S/N ratio, resolution, measurement speed and ease of use, making it ideal for countless applications.
High-End Sensitivity
The IRXross is a mid-level FTIR model that achieves high-end level S/N. It enables best-in-class low noise with P-P values of 55,000:1 for one minute of integration.
Astoundingly Low Noise
The 100 %T line was obtained by successively measuring the background and sample values without placing a sample in the sample compartment. Excluding the peaks for water vapor and carbon dioxide, the noise level (P-P value) was within ±0.005 %T, which shows that it can acquire data with low noise.
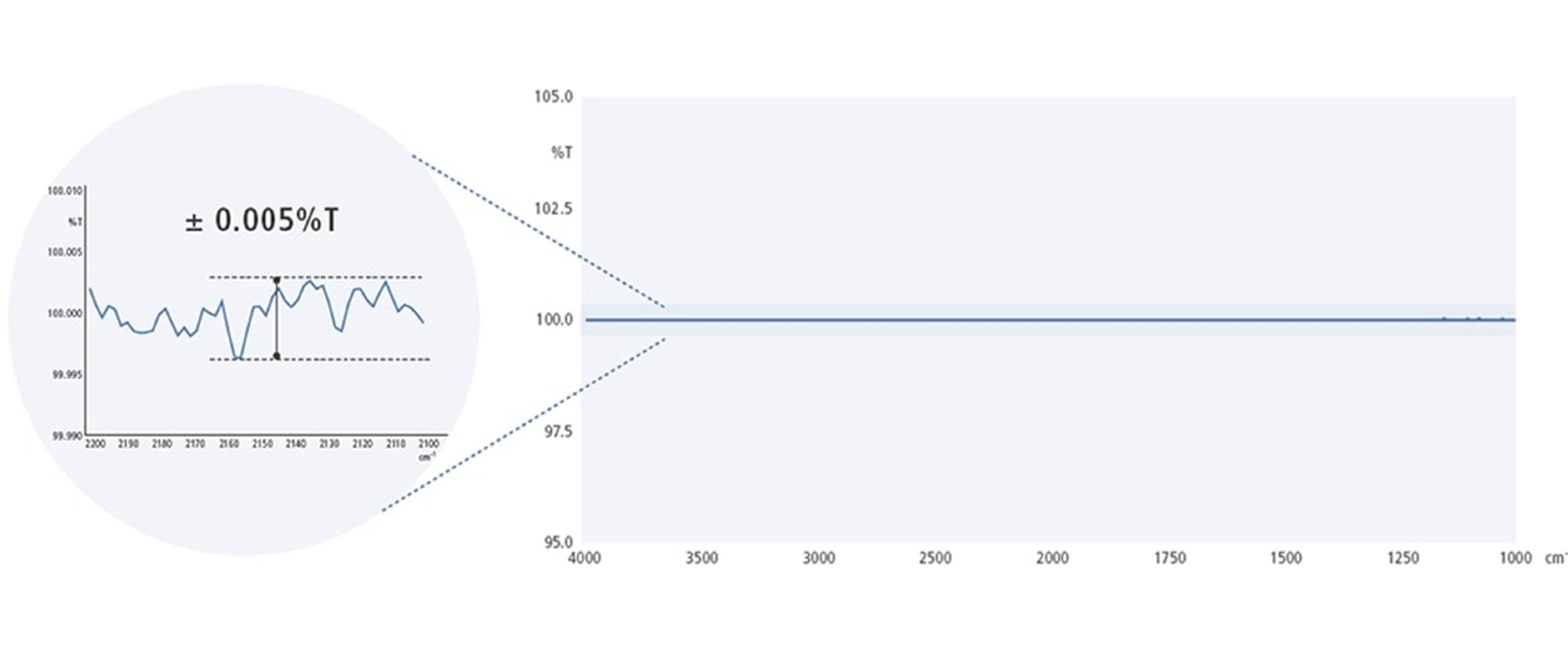
IRXross 100%T Line. Image credit: Shimadzu Scientific Instruments
0.25 cm-1 Resolution
Enables high-resolution measurements
High-Speed Measurement
A measurement speed of 20 spectra per second enables faster reaction tracking. In this example, when tracking the curing reaction in UV curable resin, the data shows that the intensity of the peak at 1,635 cm-1 began decreasing 5.0 seconds after UV irradiation and the reaction was finished by 5.5 seconds.
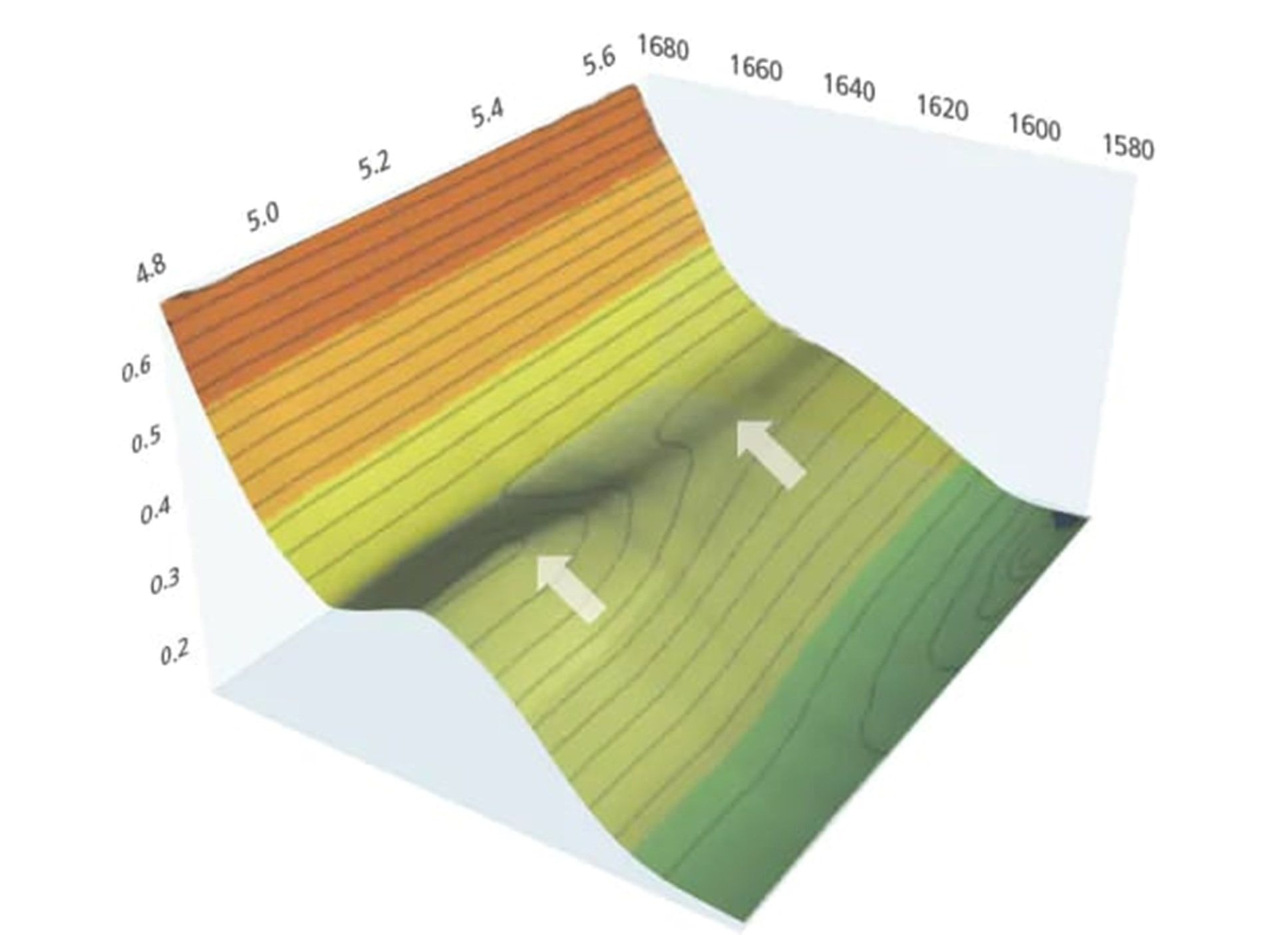
Image credit: Shimadzu Scientific Instruments
Built-in Analytical Intelligence
The system is equipped with IR Pilot™ for easy start-up and navigation. The standard package includes 23 macro application programs. Even operators unfamiliar with FTIR spectrophotometry can analyze samples easily by simply selecting the purpose of analysis and attachment they are using.
With IR Pilot, users can program the IRXross to specifically support identification testing by making pass/fail judgments for test samples based on verification methods specified in pharmacopoeia and official methods. They can also program the system to support contaminant analysis using Shimadzu’s proprietary algorithm in combination with a spectral library containing over 550 spectra for substances commonly detected as contaminants.
Software
LabSolutions™ Software DB IR and LabSolutions CS IR meet the requirements of Electronic Record and Electronic Signature regulations. In addition, the software includes built-in validation test macros for compliance with requirements from various pharmacopeia, including USP.