Jun 17 2010
HyperBranch Medical Technology, Inc. announced today that it has received a CE Mark for Adherus(TM) Spinal Sealant. Adherus(TM) Spinal Sealant is used in spinal surgeries where a water tight seal is required to prevent cerebrospinal fluid leakage from the dural repair.
Prevention of cerebrospinal fluid leaks should benefit the patient in shorter hospital stays and fewer post-surgical complications such as reduced pain and fewer infections. Adherus Spinal Sealant also acts as an adhesion barrier, thereby simplifying subsequent surgeries should the need arise. The unique, single use device is terminally sterilized and can be applied in both Open and MIS procedures through either a 100mm or 150 mm tip. The CE Mark provides regulatory approval for the company to begin sales in Europe and other countries outside the U.S.
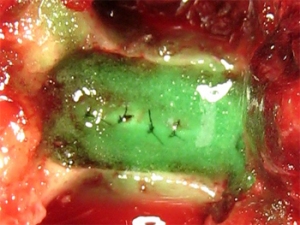 Adherus Spinal Sealant
Adherus Spinal Sealant
“Adherus(TM) Spinal Sealant is an easy to use device that complements HyperBranch’s neurosurgical sealant product line. Adherus(TM) Dural Sealant, which was first launched in 2009, is currently marketed outside the U.S. for cranial surgery. Adherus(TM) Spinal Sealant has negligible swelling, degrades slowly not only provides a watertight seal but also acts as an adhesion barrier to limit scarring, a major advantage if subsequent surgery is required,” said John Conn, President and Chief Executive Officer.
The Adherus(TM) products are synthetic hydrogels which polymerize in a moist field, flow optimally depending on the application, and are bio-degradable as the tissue re-establishes itself. The biocompatible composition is stored at room temperature and is delivered through a custom applicator to meet the specific needs of the procedure.
HyperBranch Medical Technology, Inc. has now received CE Marks for ocular, cranial, and spinal sealants as well as a hernia mesh fixation product. The Company has completed a U.S. feasibility study and is starting the Pivotal portion of the study this month for the Adherus Dural Sealant. The Spinal product will follow the same approval path in the U.S. The company’s ophthalmic sealant is currently licensed to and marketed by BD Medical.