Nov 2 2010
Oil and water don't mix, but add in some nanofibers and all bets are off.
A team of UCLA chemists and engineers has developed a new method for coating large surfaces with nanofiber thin films that are both transparent and electrically conductive. Their method involves the vigorous agitation of water, dense oil and polymer nanofibers. After this solution is sufficiently agitated it spreads over virtually any surface, creating a film.
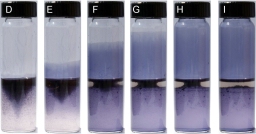 Sequence of images illustrating growth of polymer film in tubes over 35 seconds
Sequence of images illustrating growth of polymer film in tubes over 35 seconds
"The beauty of this method lies in its simplicity and versatility," said California NanoSystems Institute ( CNSI ) researcher Richard B. Kaner, a professor of chemistry and biochemistry and a professor of materials science and engineering at the UCLA Henry Samueli School of Engineering and Applied Science. "The materials used are inexpensive and recyclable, the process works on virtually any substrate, it produces a uniform thin film which grows in seconds and the entire thing can be done at room temperature."
Conducting polymers combine the flexibility and toughness of plastics with electrical properties. They have been proposed for applications ranging from printed electronic circuits to supercapacitors but have failed to gain widespread use because of difficulties processing them into films.
"Conducting polymers have enormous potential in electronics, and because this technique works with so many substrates, it can be used in a broad spectrum of applications, including organic solar cells, light-emitting diodes, smart glass and sensors," said Yang Yang, a professor of materials science and engineering at the Samueli School of Engineering and Applied Science and faculty director of the Nano Renewable Energy Center at the CNSI.
One of the potential applications is smart, or switchable, glass that can change between states when an electric current is applied — for example, switching between see-through and opaque states to let light in or block it. The UCLA research group is applying the technique to other nanomaterials in addition to polymer nanofibers in the hopes of expanding the number of available applications.
The team's solution-based technique, published in the peer-reviewed journal Proceedings of National Academy of Science, was discovered serendipitously when a transparent film of polymer spread up the walls of a container while nanofibers in water were being purified with chloroform.
"What drew me in immediately was the eerie phenomenon of what appeared to be self-propelled fluid flow," said Julio M. D'Arcy, lead author on the PNAS paper and a senior graduate student in the Kaner's UCLA lab.
"Now I can tell people that I make films in L.A.," he joked.
When water and oil are mixed, a blend of droplets is formed, creating a water–oil interface that serves as an entry point for trapping polymer nanofibers at liquid–liquid interfaces. As droplets unite, a change in the concentration of blended solids at the water–oil interface leads to a difference in surface tension. Spreading up a glass wall occurs as result of an attempt to reduce the surface-tension difference. Directional fluid flow leads to a continuously conductive thin film comprised of a single monolayer of polymer nanofibers. The uniformity of the film surface is due to the particles being drawn out of the water–oil interface, sandwiched between two fluids of opposing surface tensions.
Development of the technology is occurring in collaboration with Fibron Technologies Inc., with support from the National Science Foundation through a Small Business Technology Transfer grant. Fibron is a small company that has licensed the technology from UCLA. It was founded by Kaner, who serves as chief scientific adviser, and two of his former Ph.D. students — Christina Baker and Henry Tran, who have gone on to take leadership roles in the company.
Fibron's CEO, Christian Behrenbruch, said "working with UCLA to develop this technology has been a win-win. It enables us to access incredibly innovative people, but also, the NSF has helped enable the establishment of a formal and transparent IP releationship with the university. The good news is that this technology is moving rapidly into commercial development."
Other techniques exist for creating thin films of conducting polymers, but each technique tends to work only a limited number of applications, or they are not feasible for scaling up. A method has long been sought which would overcome the limitations of each of the previous methods. The water and oil technique, with a bit of nanotechnology thrown in, might provide just that — a scalable universal method for creating large thin films of conducting polymers.