Apr 12 2013
Kiel based research group discovers nano-crystals at the interface between two liquids. Not all liquids are mixable. Researchers from the Institute of Experimental and Applied Physics of Kiel University (CAU) have investigated chemical processes with atomic resolution at the interface between two such liquids and have made an exciting discovery.
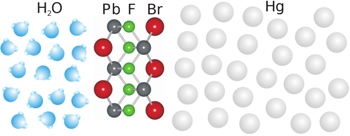 Illustration of the nano-layer at the liquid interface between the salt solution and mercury. Physicists from Kiel University discovered the formation of an ordered crystal of exactly five atomic layers between the two liquids with brilliant X-rays. Copyright/Image: CAU
Illustration of the nano-layer at the liquid interface between the salt solution and mercury. Physicists from Kiel University discovered the formation of an ordered crystal of exactly five atomic layers between the two liquids with brilliant X-rays. Copyright/Image: CAU
During an experiment carried out at Germany’s largest accelerator centre DESY (Deutsches Elektronen-Synchrotron) in Hamburg, they observed the formation of an ordered crystal of exactly five atomic layers between the two liquids, which acts as a foundation for growing even bigger crystals. The experiment was performed in cooperation with scientists from Israel, the USA, and DESY. The results have just been published in the renowned scientific journal Proceedings of the National Academy of Science. They may result in new semiconductor and nano-particle production processes.
Everyone knows that oil and water do not mix. However, how the interface between two immiscible liquids behaves on an atomic scale is almost completely unknown up to now as it cannot be investigated at this level by most modern surface science methods. Solving this final piece of the puzzle is the aim of the team of Dr. Bridget Murphy and Professor Olaf Magnussen from the Physics Department at Kiel University. To do this the scientists use the brilliant X-rays at DESY’s ring accelerator PETRA III. There the LISA diffracometer (Liquid Interfaces Scattering Apparatus), an instrument developed by the physicists from Kiel, deflects the highly focussed X-ray beam onto the liquid sample. “LISA was custom designed for investigating interfaces in liquids because here important chemical processes take place” explains Bridget Murphy, who was responsible for building up this instrument in the last few years.
In their latest work the researchers wanted to find out, for the first time, what exactly occurs during chemical growth at liquid interfaces. They investigated mercury in a salt solution containing fluorine, bromine and lead ions and obtained an astonishing result: although the molecules in both liquids were disordered, a nanometre thin layer, that is a ten thousandth of the width of a human hair, with crystalline order formed at their interface. “Our X-ray data show that this layer consists of an atomic layer of fluorine between two layers of lead and bromine”, explains team member Annika Elsen, who just received her doctorate for this work. “Subsequently, larger crystals grow perfectly aligned on top of this nano-layer crystal.”
The atomic order that develops at such disordered liquid interfaces is not only of fundamental interest for science. In fact, in the last few years, a range of chemical processes for producing materials and nano-particles has employed growth at liquid interfaces. For example, two years ago American scientists at the University of Michigan developed a similar process for manufacturing semiconductor germanium with an extremely energy efficient method from its oxide. Further developments of such processes could help to reduce the high energy costs in the production of solar cells. In order to achieve this the details of these processes, a better understanding on the atomic scale is required. The work of the Kiel scientists is a first step in this direction.
Kiel University, a research university in north Germany, has a proven international expertise in nano-science. Experiments with synchrotron radiation make an important contribution to this field. In a series of research consortia, funded by the ministry for education and research (Bundesministerium für Bildung und Forschung) Kiel scientists design and develop new methods and instruments.