Jul 11 2013
Introductory chemistry students learn that oil and water repel each other. So do other hydrophobic substances, which carry no electric charge, and hydrophilic substances, which carry an electric charge that allows them to mix with water.
In a study reported in the July 1, 2013 Angewandte Chemie, a group of bioengineers from the University of Illinois at Urbana-Champaign have found a way to strongly adhere hydrogels to hydrophobic silicone substrates, an innovation that provides a valuable new tool for microscale biotechnology. The article reporting the work was highlighted by the editors as a “Hot Paper” in Soft Material Chemistry.
Microscale biotechnologies, including cell culture platforms and biochips, have important applications in genomics, tissue engineering, and many other areas of biology. Silicone polymers are often used as a component material in these technologies because of their advantageous properties: silicones are inert, elastic, biocompatible, and easy to work with.
One major drawback of this type of material in biological applications is that surfaces formed by silicones are extremely hydrophobic. These surfaces therefore prohibit easy flow of aqueous solutions and prevent the binding of some biomolecules, while promoting undesired binding of others. Chemical modifications or coatings can be used to make the surfaces hydrophilic, but the results produced by these treatments are temporary.
The Illinois study, led by Hyunjoon Kong, assistant professor of chemical and biomolecular engineering and member of the Regenerative Biology and Tissue Engineering research theme at the Institute for Genomic Biology, addressed this problem. The group worked to develop a method to permanently modify a silicone polymer surface. Kong’s group sought a way to “glue” an alginate hydrogel, a water-absorbing substance also used in food and medical industries, to a surface formed by polydimethylsiloxane (PDMS), a silicone polymer that is a common material in microscale biotechnologies.
“It has been often suggested that integration of the silicone-based materials with organic, tissue-like hydrogel materials should enhance functionality,” says Kong. “However, it was a grand challenge to attain and sustain adhesion between two disparate materials.”
The solution, developed by first author Chaenyung Cha, Kong, and others, was to develop a multi-step protocol to covalently link alginate, the polysaccharide that is also a component of the hydrogel, to a PDMS surface. The resulting alginate-PDMS can then participate in a cross-linking reaction that forms an alginate hydrogel.
Tests performed as part of the study showed that the attachment of hydrogel to PDMS formed by this process is stable for several months, can tolerate bending and repeated stretching, and is not degraded by aqueous solutions. The hydrogel is more hydrophilic than the surfaces produced by previously developed treatments, and its physical properties can be easily controlled by modifying the crosslinking reaction used in its formation.
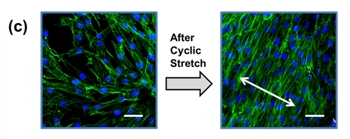
The study also explored several practical applications of the hydrogel-PDMS surface. Fibroblasts, a type of cells found in connective tissue, were first cultured on a hydrogel-PDMS surface, and then chronically exposed to repeated stretching. This type of manipulation would not be possible with less elastic cell culture substrates, and has value for researchers studying the effects of mechanical stress on living cells inside the body.
Another experiment showed that the hydrogel could be successfully and stably synthesized within the channels of a microfluidic device.
“This method will greatly advance quality of cell culture platforms and microfluidic devices. It will further benefit design of novel drug delivery systems and cell transplantation devices,” Kong said.
Research was performed in the Department of Chemical and Biomolecular Engineering and the Institute for Genomic Biology at Illinois. Cha, who worked on the study as a graduate student in Kong’s lab, is now a postdoctoral fellow at the Massachusetts Institute of Technology. Other authors of the study are Eleni Antoniadou, Minkyung Lee, Jae Hyun Jeong, Wylie W. Ahmed, Taher A. Saif, Gutgsell Professor of Mechanical Science and Engineering, and Stephen A. Boppart, who has faculty appointments in the departments of Electrical and Computer Engineering, Bioengineering, and Medicine.