Feb 25 2014
When deciding what materials to use in building something, determining how those materials respond to stress and strain is often the first task. A material's macroscopic, or bulk, properties in this area — whether it can spring back into shape, for example — is generally the product of what is happening on a microscopic scale. When stress causes a material's constituent molecules to rearrange in a way such that they can't go back to their original positions, it is known as "plastic deformation."
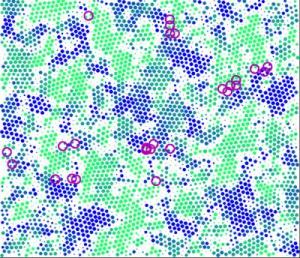 Researchers at the University of Pennsylvania have devised a method to study stress at the macro and micro scales at the same time, using a model system in which microscopic particles stand in for molecules. This method has allowed the researchers to demonstrate an unusual hybrid behavior in their model material: a reversible rearrangement of its particles that nevertheless has the characteristics of plastic deformation on the macroscale. In this illustration of a portion of the material, dots represent particle positions and circles represent rearrangements. Credit: University of Pennsylvania
Researchers at the University of Pennsylvania have devised a method to study stress at the macro and micro scales at the same time, using a model system in which microscopic particles stand in for molecules. This method has allowed the researchers to demonstrate an unusual hybrid behavior in their model material: a reversible rearrangement of its particles that nevertheless has the characteristics of plastic deformation on the macroscale. In this illustration of a portion of the material, dots represent particle positions and circles represent rearrangements. Credit: University of Pennsylvania
Researchers at the University of Pennsylvania have devised a method to study stress at the macro and micro scales at the same time, using a model system in which microscopic particles stand in for molecules. This method has allowed the researchers to demonstrate an unusual hybrid behavior in their model material: a reversible rearrangement of its particles that nevertheless has the characteristics of plastic deformation on the macroscale. That kind of plastic deformation is more akin to what happens with ketchup or toothpaste, a liquid-like flowing instead of a brittle fracturing of the material's particles.
Their study could pave the way for designing this potentially useful trait into new materials. Plastic deformation dissipates energy rather than transferring it, so a material that could repeatedly deform in this way could be used to dampen vibrations or protect against impacts.
The study was conducted by postdoctoral researcher Nathan Keim and professor Paulo Arratia, both of the Department of Mechanical Engineering and Applied Mechanics in Penn's School of Engineering and Applied Science. It was published in Physical Review Letters.
"If you're driving your car and you hit a lamppost, your car would be totally deformed," Arratia said. "That's plastic deformation because it's irreversible. When you put your car into reverse and back up away from the lamppost, it's not as if the car just springs back into shape. And even if you take it to the body shop and hammer it out to look like new, it's never going to be the same again on the atomic level."
"On the other end of the spectrum," Keim said, "there's elastic deformation. Those deformations are usually reversible rearrangements. If you take a piece of steel and deform it just a little bit, like sitting on the hood of your car rather than running it into a lamppost, it will come back exactly as it started, even on the microstructural level.
"What we were able to show with this model is that you can have something that's between the two ends of this spectrum, a hybrid regime that is plastic but also reversible."
Simultaneously investigating both the macroscopic and microscopic behavior of a material under stress time is a challenge: bulk materials are generally opaque, so seeing what is happening inside of them while they maintain their bulk properties is impossible. To provide a window to this inner world, the researchers built a model material that sacrifices complexity for access.
"The complexity we sacrificed is the third dimension," Keim said. "We have made an effectively 2-D material in the lab that consists of microscopic particles that sit on an oil-water interface. These particles have a little electric charge that keeps them constantly pushing away from each other, which means you can think of them as drops of liquid in an emulsion or even as atoms."
The researchers used this model material to study a behavior known as the "yielding transition," which is how disordered solids begin to flow. Just as toothpaste doesn't immediately flow out of the tube and ketchup doesn't pour out of the bottle once the cap is removed, the particles of a disordered solid are jammed against one another and don't easily rearrange themselves unless outside energy is added.
In the case of ketchup, this outside energy often takes the form of tapping the side of the bottle. In the case of this model material, the researchers added this energy by way of a needle that also sat at the material's oil-water interface, in the plane with the rest of the particles. Using an electromagnetic field, they could swing the needle back and forth against the particles and measure how much resistance they provided.
In experimenting with this model material, the researchers were surprised by what they described as a "learned behavior." They found that by repeatedly moving the needle back and forth, irreversibly deforming the material many times, the particles eventually rearranged themselves in such a way that they went back to their original state after each cycle of deformation.
"The material can reorganize itself so that the link between plasticity and irreversibility is broken," Arratia said. "The material flows slightly, and yet, at the end of each cycle, it is exactly unchanged."
Critically, this reversible rearrangement of particles is not like what happens in elastic deformations.
"After the material is deformed," Keim said, "it doesn't just bounce back to its original state. Rather than just pushing back elastically, like a spring would, it gives a little bit and dissipates energy, more like a viscous fluid than a solid."
Having a mix of plastic and elastic properties is potentially useful.
"You might want this in materials where the alternative to flowing is shattering," Keim said. "You'd rather that it deform a little bit before breaking, and you'd also want things not to be severely altered or damaged by being deformed again and again. This kind of plastic deformation also dissipates a lot more energy; you want the body of your car to absorb the energy of an impact and dissipate it, not transfer it to you."
While this behavior has now only been observed in the researchers' model material, understanding the conditions in which it arises could lead to ways of producing it in materials that might be used outside the lab.
"We are designing for failure," Arratia said. "Elastic deformation is pretty great, but it can't last forever, eventually something has to give. And when it gives, this would be a pretty great way to do it. You'd like that transition to be as graceful and non-destructive as possible."