May 26 2014
Analysis of a manganese-based crystal by scientists at the National Institute of Standards and Technology (NIST) and the Massachusetts Institute of Technology (MIT) has produced the first clear picture of its molecular structure. The findings could help explain the magnetic and electronic behavior of the whole family of crystals, many of which have potential for use in batteries.
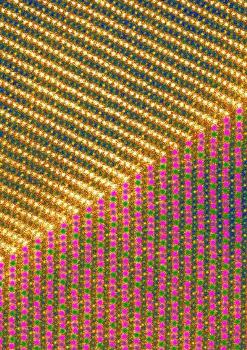 At the top of this image, sodium fills in layers of the crystal, represented by one bright yellow dot followed by three darker ones; at bottom, the layers' magnetic ordering is shown as green and purple dots representing magnesium at two different charge states, with the green-in-purple dots representing a mixture of the two charge states. Artwork generated from a scanning tunneling microscope image. (Credit: NIST)
At the top of this image, sodium fills in layers of the crystal, represented by one bright yellow dot followed by three darker ones; at bottom, the layers' magnetic ordering is shown as green and purple dots representing magnesium at two different charge states, with the green-in-purple dots representing a mixture of the two charge states. Artwork generated from a scanning tunneling microscope image. (Credit: NIST)
The family of crystals it belongs to has no formal name, but it has three branches, each of which is built around manganese, cobalt or iron—transition metals that can have different magnetic and charge properties. But regardless of family branch, its members share a common characteristic: They all store chemical energy in the form of sodium, atoms of which can easily flow into and out of the layers of the crystal when electric current is applied, a talent potentially useful in rechargeable batteries.
Other members of this family can do a lot of things in addition to energy storage that interest manufacturers: Some are low-temperature superconductors, while others can convert heat into electricity. The trouble is that all of them are, on the molecular level, messy. Their structures are so convoluted that scientists can't easily figure out why they do what they do, making it hard for a manufacturer to improve their performance.
Fortunately, this particular manganese crystal is an exception. "It's the one stable compound we know of in the manganese branch that has a perfect crystal lattice structure," says Jeff Lynn of the NIST Center for Neutron Research (NCNR). "That perfection means we can isolate all its internal electronic and magnetic interactions and see them clearly. So now, we can start exploring how to make those sodium atoms more movable."
Team members from MIT made the material and performed analysis using state-of-the-art lab techniques such as electron microscopy, but they needed help from the NCNR's neutron beams to tease out the interactions between its individual atoms. The effort showed that the crystal was unusual for reasons beyond its structural perfection. Its layers absorb sodium in a fashion rarely seen in nature: In each layer, one "stripe" of atoms fills up completely with sodium, then the next three stripes fill up only halfway before another full stripe appears. Lynn says the pattern is caused by different charges and magnetic moments that manganese atoms possess in different parts of the crystal, a feature revealed by analysis of the NCNR data.
"This particular crystal is probably not the one you'd use in a battery or some other application, it just permits us to understand what's happening with its internal structure and magnetism for the first time," Lynn says. "Now we have a basis for tailoring the properties of these materials by changing up the transition metals and changing the sodium content. We no longer have to hunt around in the dark and hope."