An international group of researchers has identified the force that makes certain crystals leap, while others break into many smaller crystals. The research team included scientists from Abu Dhabi, New York University, the National Institute for Materials Science, and the Max Planck Institute for Solid State Research.
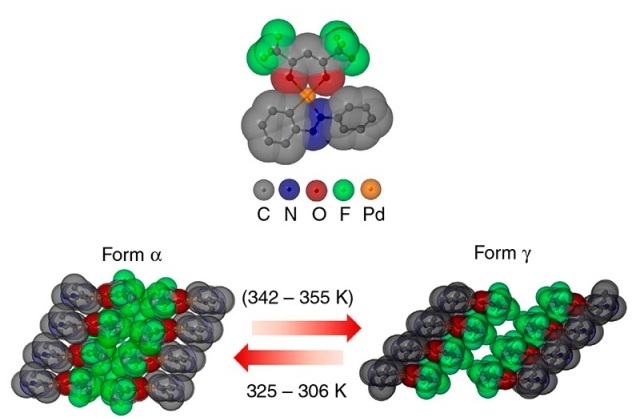 Crystalline jumping power: In the PHA molecule, organic molecule parts containing fluorine, oxygen and nitrogen are grouped around a central palladium atom. The molecule crystallises into different forms. When it switches from the alpha form to the gamma form at 342 to 355 Kelvin (69 to 82 degrees Celsius) the crystal expands very rapidly and grows substantially, causing it to jump up from the underlying surface.
Crystalline jumping power: In the PHA molecule, organic molecule parts containing fluorine, oxygen and nitrogen are grouped around a central palladium atom. The molecule crystallises into different forms. When it switches from the alpha form to the gamma form at 342 to 355 Kelvin (69 to 82 degrees Celsius) the crystal expands very rapidly and grows substantially, causing it to jump up from the underlying surface.
According to the study, the structure of the material changes when the temperature varies between 700 and 800°C. Initially, there is an increase in mechanical tension, which is discharged quickly and this makes the material to jump into the air.
This phenomenon is known as thermosalient effect, which can possibly be used in actuators and artificial muscles. On the other hand, the tension that occurs during structural changes can also break the crystals. This phenomenon can make chemical reactions more efficient.
Materials scientists are often inspired by natural systems. Some materials in plants respond to external stimuli, such as elevated humidity levels, with movement - mimicking the properties of these materials could be useful in the development of devices such as artificial muscles.
Thermosalient crystals could serve as engines for robots and micro-machines that eliminate the need for fuel supply or power sources. Such crystals are formed from the organometallic compound (phenylazophenyl) palladiumhexafluoroacetylacetonate (PHA).
Upon heating to over 700°C, these crystals leap and thus may help flex artificial muscles. The international research team has discovered the factor that causes thermosalient effect in PHA crystals.
Studying PHA crystals
During the course of their study, the researchers identified three unknown crystal forms which can be adopted by the material in addition to the two known phases. To date, PHA is the only known organometallic compound which occurs in a range of structures.
The thermosalient effect takes place between two phases, called α (alpha) and γ (gamma). The crystals expand in a single direction when PHA changes from one form into another.
However, even the fact that the size of a PHA crystal changes significantly when swapping between α and γ structures is not sufficient to make the crystal leap from the hotplate at a specific temperature. This effect is actually cause by a buildup of mechanical tension in the crystal as the structure alters.
Tomče Runčevski from Max Planck Institute for Solid State Research explained:
"After reaching a certain point, this tension then discharges 10,000 times faster than in it does in the case of structural changes in which the thermosalient effect does not occur."
Shattering into microcrystals
This build up of tension does not always cause the crystal to jump, however - there are several other effects that can be observed.
The researchers studied another type of crystal, with the systematic name (Z)-4-(4-bromobenzylidene)-2-methyloxazole-5(4H)-one, shortened by the researchers to "Z-1". When UV light is used to irradiate the crystals in the compound, Z-1 molecules will form pairs. The structure of the material in this photochemical reaction changes in such a way that the crystals break into many smaller crystals.
"Such reactions harbour potential for chemical processes in industrial applications, for example, because unlike most of the other chemical reactions, they only take place in one direction."
Tomče Runčevski
The one-way nature of this process means that the broken-up crystals will not reform into larger crystals of the precursor, making them much more flexible for use in chemical processes.
Analyzing crystal structure with advanced x-ray powder diffraction
The Stuttgart researchers were also able to explain the structure of the microcrystal product and the larger precursor crystal, and therefore map out the structural changes which occur during the photochemical reaction.
This was not an issue for the precursor, as it exists in the form of a solid crystal, or monocrystal, and conventional x-ray crystallography techniques reveal its structure. However, to inspect the structure of the powdery product, the scientists developed a new trick, which was also used in analyzing the PHA structures. The technique is a refined version of X-ray powder diffraction.
Although the techniques employed to examine monocrystals generate three-dimensional information, powder diffraction gives only one-dimensional information, which made it considerably harder to rebuild the three-dimensional structure of the crystal. However, the researchers were able to refine their analysis of the data to such an extent that three-dimensional data is also available.
This technique enabled the scientists to analyze the microcrystals in detail, including mapping the various stages of transformation in the Z-1 crystal structure over time.
"Gaining a deeper understanding of how it works could potentially help us develop materials that are suitable for practical applications in artificial muscles or actuators."
Robert E. Dinnebier
When material scientists gain a better insight into the structural transformation, they would be able to search for substances that have the preferred effect and at the same time fulfill other conditions needed for specified practical applications, whether it is for resource-efficient chemical reactions or artificial muscles.
References and further reading
Jumping Crystals - Max-Planck Institute for Solid State Research
"Following a Photoinduced Reconstructive Phase Transformation and its Influence on the Crystal Integrity: Powder Diffraction and Theoretical Study" - Runčevski et al, Angewandte Chemie 2014