Jun 11 2015
A team of researchers from the National Institute of Standards and Technology (NIST) has formulated a simple method to produce platinum "nano-raspberries”. "Nano-raspberries” are basically microscopic clusters of nanoscale particles of the valuable metal.
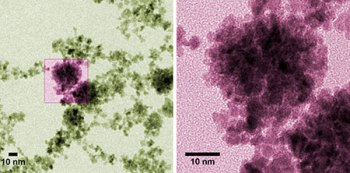 Colorized micrographs of platinum nanoparticles made at NIST. The raspberry color suggests the particles’ corrugated shape, which offers high surface area for catalyzing reactions in fuel cells. Individual particles are 3-4 nm in diameter but can clump into bunches of 100 nm or more under specific conditions discovered in a NIST study. Credit: Curtin/NIST
Colorized micrographs of platinum nanoparticles made at NIST. The raspberry color suggests the particles’ corrugated shape, which offers high surface area for catalyzing reactions in fuel cells. Individual particles are 3-4 nm in diameter but can clump into bunches of 100 nm or more under specific conditions discovered in a NIST study. Credit: Curtin/NIST
The important aspect of this method is the creation of the berry-like shape, which possesses a high surface area, therefore can be very useful in developing catalysts. Another key aspect is that the researchers are now able to explain exactly when and why the berry clusters form into bigger clusters of "nano-grapes”, which is definitely good news for industrial chemists.
Their work holds promise for creating more versatile fuel cells. The nanoparticles would behave as catalysts to facilitate conversion of methanol to electricity in fuel cells. The nano-raspberry fabrication procedure lasted only 40minutes and offers many advantages. Reactions are highly efficient as the berries feature a high surface area. Furthermore, the procedure deployed by NIST uses a benign, green solvent or water. Another advantage is that the clusters catalyze methanol reactions reliably and maintain stability at room temperature for about eight weeks.
Platinum is an expensive metal; however was used by the researchers only as a model. They hope to use the findings of their study to discover substitute catalyst materials, and understand clumping behavior in solvents.
To bind nanoparticles to an electrode in fuel cells, they are mixed with solvents. For the first time the researchers measured particle clumping in four distinct solvents, to comprehend how these formulas impact particle properties. In the case of liquid methanol fuel cells, catalyst particles should not be clumped but be separated and spread in the liquid.
"Our innovation has little to do with the platinum and everything to do with how new materials are tested in the laboratory," project leader Kavita Jeerage says. "Our critical contribution is that after you make a new material you need to make choices. Our paper is about one choice: what solvent to use. We made the particles in water and tested whether you could put them in other solvents. We found out that this choice is a big deal."
The NIST researchers studied and measured the conditions which caused the platinum particles measuring 3 to 4nm in diameter to clump together into bunches measuring 100nm wide or more. They discovered that the electrical properties of the solvent impacted the clumping. The raspberries tend to form larger grape bunches in solvents which are less "polar”, meaning that the solvent molecules was deficient in regions with very strong positive or negative charges. For instance water is said to be a highly polar molecule. This was predicted by the team; however they did not realize that the trend does not scale predictably.
Water, methanol, ethanol and isopropanol were the four solvents chosen for the experiments in the order of reducing polarity. In methanol, agglomeration was minimal, with the clusters becoming around 30% bigger than they were in water. In the case of ethanol and isopropanol, the clusters were enormous bunches becoming around 400% and 600% larger, respectively. For catalytic purposes, this would be low suspension quality.
Since the nanoparticles clustered slowly and not significantly in methanol, the researchers were convinced that the particles could be shifted to that solvent, with the assumption that they would be used in a few days. Hence an expiration date was set on the catalyst.
Two college students under the NIST's Summer Undergraduate Research Fellowship (SURF) program assisted the research team in gathering extensive data required for the study.