Sep 8 2015
A research team at the Rice University has developed solar-based water-splitting technology that uses light-activated nanoparticles.
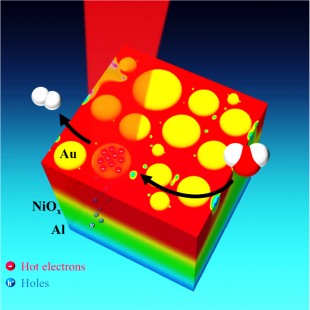 Rice University researchers have demonstrated an efficient new way to capture the energy from sunlight and convert it into clean, renewable energy by splitting water molecules.
Rice University researchers have demonstrated an efficient new way to capture the energy from sunlight and convert it into clean, renewable energy by splitting water molecules.
Researchers have devised a new and efficient method for capturing solar energy and converting it into renewable, clean energy by splitting of water molecules.
The newly found technology, which has been explained in Nano Letters – a journal published by the American Chemical Society, is based on an arrangement of light-activated gold nanoparticles used for capturing sunlight and transmitting the solar energy to high-excitation electrons referred to by scientists as hot electrons.
“Hot electrons have the potential to drive very useful chemical reactions, but they decay very rapidly, and people have struggled to harness their energy,” said lead researcher Isabell Thomann, assistant professor of electrical and computer engineering and of chemistry and materials science and nanoengineering at Rice.
“For example, most of the energy losses in today’s best photovoltaic solar panels are the result of hot electrons that cool within a few trillionths of a second and release their energy as wasted heat.”
Prior to cooling if these hot electrons are trapped, providers of solar-energy will be able to increase their solar-to-electric power-conversion efficiencies substantially. Such high conversion efficiencies could help meet the national goal of bringing down solar electricity costs.
Thomann and her team conducted the studies on the light-activated nanoparticles at Rice’s Laboratory for Nanophotonics (LANP). During the experiment, the light was captured by these nanoparticles and converted into plasmons, which are electron waves flowing across the metal surface of the nanoparticles like a fluid. These plasmons exist at high-energy states for very short durations, however, the research team at Rice and other places have been successful in capturing the energy from plasmons and converting it into useful forms of heat or light energy. The plasmonic nanoparticles are one of the most efficient methods of capturing the energy of hot electrons, and the research team at LANP has shown substantial progress in this energy capture in many of their recent studies.
Graduate students Shah Mohammad Bahauddin, Hossein Robatjazi and Chloe Doiron are part of the research team headed by Thomann. They have devised a system for utilizes the energy from hot electrons for splitting water molecules into hydrogen and oxygen. This splitting of water molecules is significant because hydrogen and oxygen are raw material for power supply in fuel cells. Fuel cells are electrochemical devices that are a clean and efficient way of producing electricity.
Before harnessing hot electrons, the team has to figure out a way to separate them from their corresponding “electron holes”. Electron holes are the low-energy states that these hot electrons move out of upon receiving the plasmonic jolt of energy. The short-life of hot electrons may be attributed to the strong inclination to return to their original, low-energy states by releasing energy.
A system that immediately separates hot electrons from electron holes is the only way to harness them before they return to their low-energy states. Electrical engineers achieve this by driving the hot electrons beyond an energy threshold, which is like a one-way valve. In spite of the underlying inefficiencies of this method, it appealed to engineers because it is based on Schottky barriers – a popular, tried-and true concept in electrical engineering.
“Because of the inherent inefficiencies, we wanted to find a new approach to the problem,” Thomann said. “We took an unconventional approach: Rather than driving off the hot electrons, we designed a system to carry away the electron holes. In effect, our setup acts like a sieve or a membrane. The holes can pass through, but the hot electrons cannot, so they are left available on the surface of the plasmonic nanoparticles.”
The setup consisted of three different layers of materials – the bottom layer was a thin sheet of shiny aluminium, the middle layer was a thin coating of transparent nickel-oxide, and the top layer was a configuration of plasmonic gold nanoparticles. The gold nanoparticles were puck-shaped disks with a diameter of 10-30nm.
These disks receive sunlight directly or as a reflection from the aluminium layer. Upon receiving sunlight, they convert the incident light energy into hot electrons. The electron holes that result from the excitation are absorbed by aluminium, which later pass through the nickel oxide layer. The nickel oxide layer does not allow hot electrons to pass through; as a result they remain on the gold layer.
This setup is then covered with water, thus allowing gold nanoparticles to play the role of catalysts that aid the splitting of water molecules. The research team measured the value of photocurrent that was available for the splitting of water molecules instead of a direct measurement of oxygen and hydrogen gases that were produced due to water splitting. Thomann added that the experimental results needed further investigations.
“Utilizing hot electron solar water-splitting technologies we measured photocurrent efficiencies that were on par with considerably more complicated structures that also use more expensive components,” Thomann said. “We are confident that we can optimize our system to significantly improve upon the results we have already seen.”
Bahauddin is pursuing physics and astronomy for his graduation, Robatjazi is pursuing electrical and computer engineering, and Doiron is pursuing applied physics for her graduation. The Welch Foundation and a National Science Foundation CAREER Award together supported the research work.