Feb 1 2016
Envisage a polymer that has removable parts and can be environment-friendly, and then can be chemically recreated to function again; alternatively, a polymer that is capable of lifting weights, and expanding and contracting, similar to muscles.
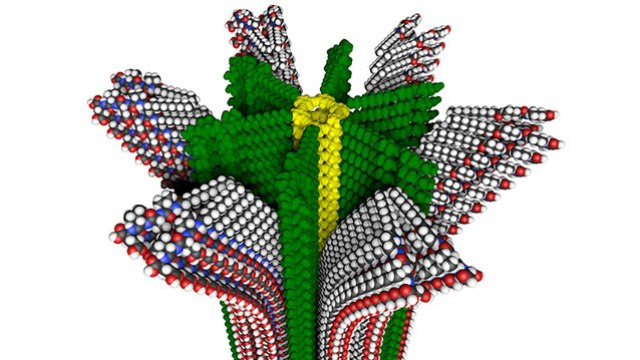 Northwestern University researchers have developed a new hybrid polymer with removable supramolecular compartments, shown in this molecular model. (Credit: Mark E. Seniw, Northwestern University)
Northwestern University researchers have developed a new hybrid polymer with removable supramolecular compartments, shown in this molecular model. (Credit: Mark E. Seniw, Northwestern University)
These tasks require polymers to have soft nano-sized and rigid compartments with varying properties, which are organized in specific ways. Northwestern University researchers have developed a new hybrid polymer that can be used in life-like materials or artificial muscles; for drug delivery or delivery of biomolecules or chemicals; in materials having self-repair ability; and for replaceable energy sources.
We have created a surprising new polymer with nano-sized compartments that can be removed and chemically regenerated multiple times. Some of the nanoscale compartments contain rigid conventional polymers, but others contain the so-called supramolecular polymers, which can respond rapidly to stimuli, be delivered to the environment and then be easily regenerated again in the same locations. The supramolecular soft compartments could be animated to generate polymers with the functions we see in living things.
Samuel I. Stupp, Materials Scientist
Stupp is director of Northwestern University’s Simpson Querrey Institute for BioNanotechnology. He is an expert in nanoscience and supramolecular self-assembly, which is the approach used in biology for creating functional ordered structures.
The hybrid polymer combines two kinds of known polymers; those that have strong covalent bonds, and those that formed with fragile non-covalent bonds, referred to as “supramolecular polymers.” The combined polymer offers two “compartments” with which materials scientists and chemists can work to present useful features.
The study has been reported in the January 29 issue of Science, under the title “Simultaneous covalent and noncovalent hybrid polymerizations.”
Our discovery could transform the world of polymers and start a third chapter in their history: that of the ‘hybrid polymer.’ This would follow the first chapter of broadly useful covalent polymers, then the more recent emerging class of supramolecular polymers. We can create active or responsive materials not known previously by taking advantage of the compartments with weak non-covalent bonds, which should be highly dynamic like living things. Some forms of these polymers now under development in my laboratory behave like artificial muscles.
Samuel I. Stupp, Materials Scientist
Polymers get their features and power from their nanoscale structures. The covalent rigid bond of Stupp’s first polymer has a cross-section shaped similar to a ninja star – a hard core with arms stretched out. The softer “life force” material is between the arms. This area can be animated, recharged and refreshed - features that can be useful in many valuable applications.
The fascinating chemistry of the hybrid polymers is that growing the two types of polymers simultaneously generates a structure that is completely different from the two grown alone. I can envision this new material being a super-smart patch for drug delivery, where you load the patch with different medications, and then reload it in the exact same compartments when the medicine is gone.
Samuel I. Stupp, Materials Scientist
Stupp is also the Board of Trustees Professor of Materials Science and Engineering, Chemistry, Medicine and Biomedical Engineering, and holds positions in Northwestern University Feinberg School of Medicine, the Weinberg College of Arts and Sciences, and the McCormick School of Engineering and Applied Science.
Stupp and his team also found out that the covalent polymerization, which is responsible for forming the rigid compartment, gets “catalyzed” by the supramolecular polymerization, yielding polymers with a higher molecular weight.
The strong-bonded covalent compartment presents the structure, whereas the weak-bonded supramolecular compartment can be used up or can wear away, depending on its function, and then can be recreated with the addition of small molecules. After the concurrent polymerizations of non-covalent and covalent bonds, the two compartments are bonded to each other, to yield a long, perfectly shaped cylindrical filament.
In order to understand the hybrid’s underlying chemistry better, Stupp and his research team collaborated with George C. Schatz, a well-known theoretician and a Charles E. and Emma H. Morrison Professor of Chemistry at the Northwestern University. Schatz’s computer simulations demonstrated that the two compartments are well integrated with hydrogen bonds – bonds which can be broken. Schatz is a co-author of the study.
This is a remarkable achievement in making polymers in a totally new way – simultaneously controlling both their chemistry and how their molecules come together. We’re just at the very start of this process, but further down the road it could potentially lead to materials with unique properties – such as disassembling and reassembling themselves – which could have a broad range of applications.
Andy Lovinger,Materials Science Program Director, National Science Foundation
The research received support from the National Science Foundation (grant DMR-1508731), the Department of Energy’s Biomolecular Materials Program (grant DE-FG02-00ER45810) and the Department of Energy’s EFRC Center for Bio-Inspired Energy Science, headquartered at Northwestern and directed by Stupp (grant DE-SC0000989).
Besides Stupp and Schatz, other authors of the paper are Zhilin Yu (first author), Faifan Tantakitti, Tao Yu and Liam C. Palmer, all from Northwestern.