Jul 12 2016
Gels are present in a variety of products used on a daily basis, but it has not been known how gels get their unique solid properties. For example, it is still a mystery why particles in the gel do not move freely, like they would in a liquid medium. For decades, scientists have been struggling to find answers to these questions.
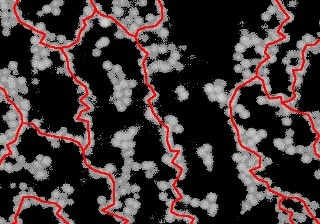 An image of a colloidal gel taken with a confocal microscope. Directed chains of particles (shown in red) that span the whole system are required for gels like this to form. (Image: Ronja Capellmann/Michael Schmiedeberg)
An image of a colloidal gel taken with a confocal microscope. Directed chains of particles (shown in red) that span the whole system are required for gels like this to form. (Image: Ronja Capellmann/Michael Schmiedeberg)
Now, researchers from FAU and Heinrich Heine University Düsseldorf have demonstrated that this property of gels is the result of directed chains of particles present in their network-like structure. The results of the study have been reported in the Nature Communication journal.
In the latest study, a unique model system was examined by the research team. This system contained a gel made up of a combination of colloids and small macromolecules called polymers. Colloids are particles that measure just a thousandth of a millimeter in size. At the outset, all of the particles were able to move freely before the liquid mixture turns into a gel. However, colloids are known to repel each other, and if they are very close to one another, so close that even the tiniest polymers is not be able to move between them, then they are pushed even closer together. This leads to the formation of colloid chains, and a gel is ultimately formed when these chains create a complicated network over the entire system. This was the phenomenon that was previously believed by researchers.
Conversely, the research team from Düsseldorf and Erlangen recently discovered that the particle chains should exhibit a certain structure to form a gel and that they must be directed. This means that there should not be any loops in the system. To put it in simpler terms, when moving via a system along a directed chain, one would need to move only in a single direction, while in the case of a system with loops one would have to return back at certain points. Directed chains provide much more stability to the system as opposed to loops and also contribute to the solid properties of a gel.
The study results are very critical to gain a better understanding about the gels’ material properties. Gels are used in a wide range of products such as gelatine, toothpaste, and other food and cosmetic products to stabilize them.
We also demonstrated that gels tend to contract as soon as there are chains of particles that stretch through the whole system. This knowledge could allow production processes for food to be improved even further.
Prof. Dr. Michael Schmiedeberg, Institute of Theoretical Physics