Jul 18 2016
Researchers from Lomonosov MSU in partnership with German researchers from the Institute of Polymer Research in Dresden (Leibniz Institute) have discovered a molecule, which they claim can offer the momentum required for the development of organic electronics. The research findings were published in Advanced Materials.
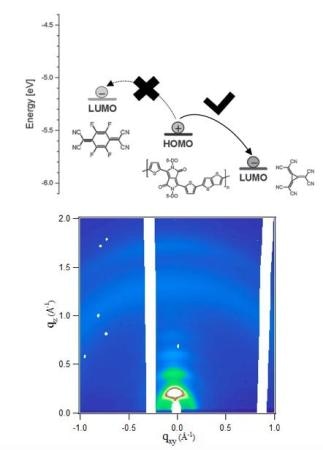 The energy levels of the studied systems and a synchrotron X-ray diffractogram measured on a thin film of an organic semiconductor doped with a derivative of [3]-radialene. (Photo credit: The Lomonosov Moscow Stte University)
The energy levels of the studied systems and a synchrotron X-ray diffractogram measured on a thin film of an organic semiconductor doped with a derivative of [3]-radialene. (Photo credit: The Lomonosov Moscow Stte University)
The team found that a derivative of [3]-radialene, a molecule identified about three decades ago, can be used to construct organic semiconductors. Dmitry Ivanov, the Head of the Laboratory of Materials Engineering at the Department of Fundamental Physics and Chemical Engineering, Moscow State University, believes that the feat will significantly add to the development of organic electronics and, more specifically, to fabrication of organic light discharging diodes and new types of organic solar cells.
Organic or "plastic" electronics evolved around 15-20 years ago and is a comparatively new scientific field.
Its function is the advancement of electronic gadgets based on organic materials. This type of gadgets are still substandard compared to the standard silicon-based electronics in relation to performance and durability. However, there are some advantages such as flexibility, lightness, transparency, thinness, and most notably -- plastic electronics is a lot cheaper than silicon.
Solar cells are one of the key applications of organic electronics. These cells will be low-cost than the silicon-based modules. The cost factor hinders silicon-based modules from being used to cover large areas, which in turn prevents maximum usage of the solar energy.
Furthermore, organic electronics can be applied to design organic field-effect transistors and light emitting devices. The molecule referred to here is the so-called dopant. When it is added to a semiconducting polymer, it greatly enhances its electrical conductivity.
The dopants for inorganic semiconductors have already been extensively used for many years, however for organic conductors, this field has not been well researched, according to one of the paper’s co-authors, Dmitry Ivanov. Presently, the most frequently used are fluorinated dopants.
When combined with diverse organic semiconductors they can significantly enhance their electrical conductivity, however some of today’s polymers that are used today in "plastic" electronics are not suitable.
Together with our Drezden colleagues we decided to design a completely new type of low molecular weight dopant for the organic semiconductor. And here it was important to choose a molecule that it was not only suitable in its energy levels, but, importantly, the dopant must be well mixed with the polymer, so that in contact with the polymer it does not segregate in a separate phase, eventually crystallizing and, in fact, losing contact with the polymer.
Dmitry Ivanov
The chief contribution of Ivanov's laboratory in this research comprised of studying the physics of the phase transitions, physics of integration in such binary systems, in the other words -- in locating an ideal candidate in relation to polymer physics.
A derivative of [3]-radialene became the chosen candidate. This is a small planar molecule possessing carbon atoms that are linked to develop a triangular structure.
The [3]-radialene possesses the most energetically ideal LUMO level, i.e. the least unoccupied molecular orbital, among other probable compounds. The electrons, with its help, can be effortlessly extracted from the semiconducting polymer matrix, thereby becoming free charges and enhancing the conductivity of the doped material. Thus the [3]-Radialene turns out to be the strongest dopant for the organic semiconductor beating all others known in the scientific literature.
All experiments with the [3]-radialene, verified by the results of quantum-chemical calculations, indicate that the substance thoroughly blends with a semiconducting polymer and facilitates increase in the polymer’s electrical conductivity by many tens and even hundreds of times.
It has been discovered that in nearly 50% of the dopant's content in the polymer, the phase separation did not happen, but the polymer’s crystalline structure slowly changed. This revealed that the dopant molecules are added into the polymer crystalline lattice and form the so-called co-crystal. And according to Ivanov, the creation of co-crystals, is precisely one of the key reasons for the new compound’s high efficiency.
The illustrated new dopant, as well its fluorinated and presently the most accepted analogues, belongs to the group of electron-deficient organic dopants, Dmitry Ivanov tells. 'Fluorine substituents are known to strongly pull the electrons away from the central part of the molecule, which increases the whole conductivity of the doped polymer. In the present work, the chemical structure of the dopant is completely different, and, in fact, appears to be even better. Perfect mixing of our dopant with the polymer matrix is, I think, the key to its performance. This could pave the way to fabrication of new organic solar cells with improved characteristics. We also think about production of organic field-effect transistors. I think it will give a significant boost to the development of organic electronic devices.'