Jul 29 2016
For decades researchers have been exploring various ways to force hydrogen into a metallic state. In materials science, a metallic state of hydrogen is considered as a holy grail as it could be used for superconductors - materials that do not possess any resistance to the flow of electrons - which improves electricity transfer efficiency significantly.
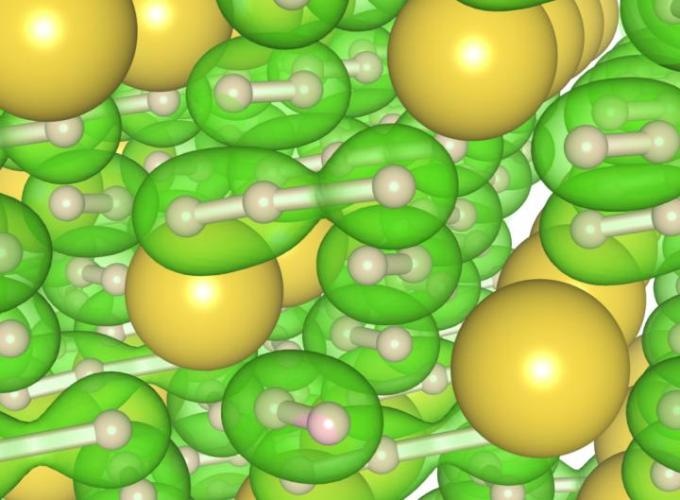 At center, in green, is the new three-atom hydrogen 'chain.' It is surrounded by several 'normal' two-atom molecules of hydrogen, also in green. The new chain configuration appears in the new material NaH7, which was produced under high pressure and high temperature conditions. The new material could change the superconductivity landscape and be useful for hydrogen storage in hydrogen fuel cells. (Photo credit: Image courtesy Duck Young Kim)
At center, in green, is the new three-atom hydrogen 'chain.' It is surrounded by several 'normal' two-atom molecules of hydrogen, also in green. The new chain configuration appears in the new material NaH7, which was produced under high pressure and high temperature conditions. The new material could change the superconductivity landscape and be useful for hydrogen storage in hydrogen fuel cells. (Photo credit: Image courtesy Duck Young Kim)
A team of researchers headed by Carnegie’s Viktor Struzhkin have experimentally developed a new group of materials by mixing hydrogen with sodium. This could revolutionize the superconductivity landscape and could be used for hydrogen-fuel cell storage.
Their research has been published in Nature Communications.
It had been estimated that some hydrogen-rich compounds that possess several atoms of hydrogen with alkali metals such as potassium, lithium, or sodium, could offer a new chemical approach to modify the electronic structure of the compound. This could pave the way to metallic high-temperature superconductors.
The challenge is temperature. The only superconductors that have been produced can only exist at impractically cold temperatures. In recent years, there have been predictions of compounds with several atoms of hydrogen coupled with alkali metals that could exist at more practical temperatures. They are theorized to have unique properties useful to superconductivity.
Viktor Struzhkin, Carnegie
Recently, those estimations have been confirmed. The Struzhkin team included Carnegie researchers Duck Young Kim, Elissaios Stavrou, Takaki Muramatsu, Ho-Kwang Mao, and Alexander Goncharov, with researchers from other institutions.
The team used theory to direct their experiments and measured the samples using a technique (X-ray diffraction) that reveals the atomic structure, and a technique (Raman spectroscopy) that identifies molecules by properties, such as their minute vibrations and rotations. In theory, the sodium/hydrogen material under pressure would remain stable, possess metallic features and unique structures, and display superconducting properties.
The team performed high-temperature/high-pressure experiments. Matter under these severe conditions can morph into new structures possessing new properties. They squeezed sodium and lithium samples in a diamond anvil cell to great pressures and using a laser they heated the samples.
At pressures between 30 - 40 GPa and temperatures of approximately 2000 K, they noticed structures of “polyhydrides,” sodium with three hydrogen atoms (NaH3) and sodium with seven atoms of hydrogen (NaH7 ) —in very rare configurations. Three negative charged hydrogen atoms in the NaH7 material were in a sequence and resembled 1D hydrogen chains, which is a new phase that is extremely different from pure hydrogen.
This configuration was originally predicted to exist in 1972, more than 40 years ago. It turns out that our experiments are in complete agreement with the theory, which predicted the existence of NaH3. The bonus is that we also observed the compound with seven hydrogen atoms.
Duck Young Kim, Carnegie
Struzhkin reflected, “Further work needs to be done to see if materials in this class can be produced at lower temperatures and pressures. But this new class of matter opens up a whole new world of possibilities.”