Oct 17 2016
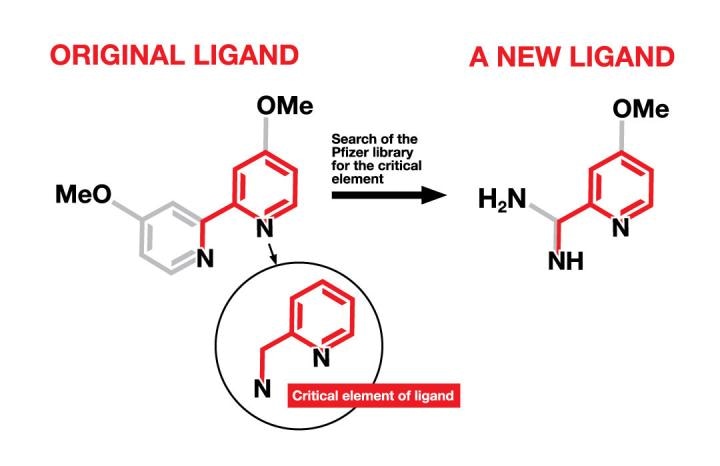 A critical element, found in an original ligand, was the basis for identifying new ligands from among a library of compounds at Pfizer originally developed for drug discovery. The new ligands will be used in the synthesis of new pharmaceutical drug candidates. (Me=methyl; O=oxygen; H=hydrogen; N=nitrogen) (Graphic by Julia Joshpe/University of Rochester)
A critical element, found in an original ligand, was the basis for identifying new ligands from among a library of compounds at Pfizer originally developed for drug discovery. The new ligands will be used in the synthesis of new pharmaceutical drug candidates. (Me=methyl; O=oxygen; H=hydrogen; N=nitrogen) (Graphic by Julia Joshpe/University of Rochester)
A group of researchers including Daniel Weix, a chemistry professor at the University of Rochester, has developed a technique to identify new catalysts that may help to synthesize drugs inexpensively and more efficiently. The trick was to attempt something that has not been tried earlier – to analyze libraries of drugs in order to find the remedy for bad chemistry: new catalysts.
The research was performed in association with Pfizer as part of a group of pharmaceutical companies concerned in finding ways to create drugs with cheap and less rare metal catalysts. These pharmaceutical companies are concerned with the use of precious metal catalysts as they are precious, expensive, and only limited quantities are available.
If a single mine closes somewhere in the world, a company might not be able to get the catalyst they need to make a life-saving drug. Using nonprecious metal catalysts, like nickel, iron, and copper, avoids this potential catastrophe.
Daniel Weix, Chemistry Professor, University of Rochester
However, there is a major hurdle when using nonprecious metal catalysts to make a life-saving drug - some of the techniques learned with precious metal catalysts may not be applicable. Specifically, metal catalysts normally contain an organic molecule that can influence the reactivity of the metal: a ligand.
“For the precious metal catalysts, we have hundreds, even thousands of ligands,” notes Weix, “but many of them do not work well for nonprecious metals.”
This problem became a focal point when Weix began to receive industrial inquiries regarding better conditions for the cross-electrophile coupling chemistry that involves the cooperation of two molecules with the help of a metal catalyst.
There were a few types of molecules that just didn’t couple well, yet were important to a wide variety of companies.
Daniel Weix, Chemistry Professor, University of Rochester
The companies had already checked their ligand libraries and identified no new ligands that could enhance the reactions further than a ligand that was reported almost four years ago by the Weix group.
The team of researchers at Pfizer, having used up the commercial supply of ligands, has suggested that the search for new ligands can be carried out among the Pfizer library that contains of 2.8 million compounds. In order to search more efficiently for such a large library, the researchers identified the least critical elements of the finest ligands and used that as a starting point to search the library for compounds with the key elements.
The search allowed researchers to retrieve more than 1,500 results and led them to carry out a more focused search about the availability as well as the likelihood of being a ligand. The search also provided the researchers a more attainable 82 results. Nine ligands in three separate classes were found to be very effective among that group. Further enhancement of the “hit” molecules has produced even better ligands.
While the new ligands look a lot like the old ligands, they were in a ‘blind spot’ for chemists. These types of ligands had never been applied in catalytic reactions, and the only metal complex we found was made by accident.
Daniel Weix, Chemistry Professor, University of Rochester
Weix thought that since these structures are commonly found in drugs, it made sense that they discovered them in the library.
Discovering new ligands is already interesting, but what matters is that how well these ligands work along with the catalysts in producing pharmaceutical compounds. Using the new ligands, the researchers solved the challenging classes of reactions that inspired the study. The improvements were dramatic in some cases, where reactions that provided no product with the old ligands offered more than 50% yield with the new ligands.
“We’ve shown that it’s worthwhile to navigate known libraries of pharmaceutical compounds,” said Weix. “What we did was only the beginning, and it’s our hope that other chemists and pharmaceutical companies will carry out similar investigations of their compound libraries.”
The research findings have been published in the Nature Chemistry journal.