Oct 19 2016
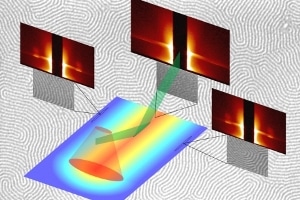 A schematic representation of the experimental and measurement setup for block copolymer phase segregation. Credit: Alan Jacobs
A schematic representation of the experimental and measurement setup for block copolymer phase segregation. Credit: Alan Jacobs
Controlling all aspects of a material in an accurate manner even at the nanoscale is greatly significant in a wide range of applications.
Block copolymers (BCPs), a type of such novel materials, are currently being developed to facilitate continued enhancement of data archiving and advanced drug refinement using protein filters, among a variety of other things.
BCPs are developed by blending materials such as water and oil, which do not readily blend in nature but can be forced to mix through a chemical “marriage” of blocks in a polymer chain. A team of Cornell engineers headed by Mike Thompson, M.A. ’82, Ph.D. ’84, and Chris Ober, professors of materials science and engineering, are investigating the possibility to monitor such novel materials by capturing the initial stages of structure development with the help of laser induced ultra-fast heating and cooling.
Their paper, “Kinetics of Block Copolymer Phase Segregation During Sub-millisecond Transient Thermal Annealing,” was published in the American Chemical Society journal, Macromolecules, on Aug. 22. Alan Jacobs, a graduate student in Thompson’s lab, is lead author.
Thompson stated that heating these materials for extremely short time periods, between 250 μs and 10 ms, enables researchers to work under temperatures in excess of 1,000 degrees even in the case of thermally sensitive polymer materials.
This opens up a whole new regime for studying the dynamics of polymer motion. Normally we think of polymers as being fairly rigid. But when you go to high enough temperatures, they become exceedingly mobile and flexible in a liquid-like state. With laser induced heating, we can reach these temperatures fast enough, and also cool down fast enough, that the block copolymers can reorder and restructure themselves into useful and interesting structures before they begin to burn.
Mike Thompson, Engineer, Cornell
BCPs order themselves according to the chemistry of their constituent “blocks,” generally known as A’s and B’s. Since both the blocks do not intend to mix, these materials attempt to detect structures where mixing is minimized.
“If you have approximately the same amount of the A and B materials,” Thompson said, “they form layers of A and B. … All the A blocks try to get next to each other, as do all the B blocks.”
However, by rapid heating and cooling, these blocks that do not usually get along are forced together by thermal motion, producing unique patterns and materials.
With enough energy in there, they will mix randomly and create a more homogeneous structure at high temperature. Then, as they cool, they are free to adopt the new and unique structures.
Alan Jacobs, Graduate Student, Cornell
In work demonstrated at the Cornell NanoScale Science and Technology Facility and the Cornell Center for Materials Research and analyzed at the Cornell High Energy Synchrotron Source, the researchers heated a BCP built from polymethyl methacrylate (PMMA) and polystyrene (PS) by using a laser spike annealing apparatus for temperatures ranging up to 550ºC (more than 1,000ºF) for durations that are not more than 10 ms.
During the very short anneal time, the material did not degrade but instead was placed in a state of controlled disorder, which could be adjusted by varying both the peak temperature and the duration of the heating. This controlled disorder, even though counterintuitive, generates a final material that has very less defects and unique characteristics, which can be designed by the annealing and the underlying chemistry.
The team is capturing a moment in time by striking the BCP with a “bolt of lightning.” Future research focuses on understanding the details related to the process.
We’ve shown that we can get fewer defects using this really rapid heating to initially do phase segregation, but we don’t yet know why, but this gives us a good start, a good idea of what’s going on in those initial stages.
Alan Jacobs, Graduate Student, Cornell
The research received grants from the U.S. Department of Defense, the National Science Foundation and the National Institutes of Health.