Nov 24 2016
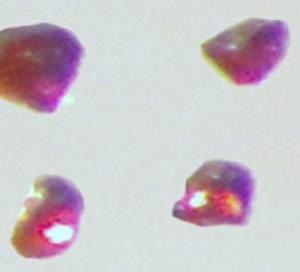 Cesium platinide hydride, or 4Cs2Pt.CsH, forms a translucent ruby red crystal and can exist only in an inert environment similar to conditions that exist in outer space. Credit: Ames Laboratory, US Department of Energy.
Cesium platinide hydride, or 4Cs2Pt.CsH, forms a translucent ruby red crystal and can exist only in an inert environment similar to conditions that exist in outer space. Credit: Ames Laboratory, US Department of Energy.
Researchers from the U.S. Department of Energy’s Ames Laboratory have been credited with the development of the first intermetallic double salt with platinum.
Materials scientists Anja-Verena Mudring and Volodymyr Smetana have successfully created and accurately characterized cesium platinide hydride (4Cs2Pt⋅CsH), a compound that can exist only in an inert environment under conditions similar to those in outer space. A translucent ruby red crystal is formed by this compound. It is the latest member of a rare family of compounds where a truly negatively charged ion is formed by a metal.
It’s a compound that as a researcher you have trouble envisioning that it can even exist, but once you do have it and can analyze it, it’s nothing like what you expect. Instead of creating a gray, shiny alloy as typically observed for many hydrogen storage materials by reacting the metals cesium and platinum with hydrogen, these red crystals form. They are really quite beautiful.
Anja-Verena Mudring, Materials Scientist, Ames Laboratory
The interesting new compound was originally extracted from cesium melt, and is highly unstable as the platinum in the compound returns to its elemental state upon oxygen exposure. From the time it was first discovered, it took a long time to gain insights into its true nature and establish its composition.
Researchers proved its existence by combining single crystal studies with powder x-ray diffraction, deep theoretical investigations, and solid-state nuclear magnetic resonance. The strong influence of relativistic effects on both platinum and cesium better explains the unusual characteristics and structure of cesium platinide hydride, which are much different from that of common intermetallic hydrides.
It’s unique. It’s the first example we have of a salt with so strongly negatively charged metal ions. Moreover, you mix an alloy with a salt and get another non-conducting salt. This allows for some deep insight into the nature of chemical bonding - and as Goethe wrote, ultimately what holds the world and its compounds together in its inmost form.
Anja-Verena Mudring, Materials Scientist, Ames Laboratory
The study results are further discussed in a paper reported in and featured on the cover of the current issue of Angewandte Chemie under the title of “Cesium Platinide Hydride 4Cs2Pt⋅CsH: An Intermetallic Double Salt Featuring Metal Anions.”