Dec 8 2016
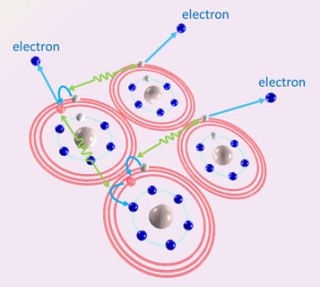 Scientists have elucidated a surprising novel mechanism of cascading electron emission caused by the Coulombic interaction between highly excited atoms. Credit: KIYONOBU NAGAYA, DEPARTMENT OF PHYSICS, KYOTO UNIVERSITY
Scientists have elucidated a surprising novel mechanism of cascading electron emission caused by the Coulombic interaction between highly excited atoms. Credit: KIYONOBU NAGAYA, DEPARTMENT OF PHYSICS, KYOTO UNIVERSITY
Scientists are working toward innovative techniques to break down electrons from matter, and this research could have an impact on radiation therapy.
When a small cluster of neon atoms is exposed to a very short and intense burst of extreme ultraviolet light, an innovative mechanism producing a huge number of ions and electrons is initiated.
A group of scientists headed by Kiyoshi Ueda, physical chemist from the Tohoku University, employed a free electron laser (FEL) at the SPring-8 Compact SASE Source test accelerator in Japan to analyze the way in which electrons are “knocked out” from the neon atom clusters.
By directing intense extreme ultraviolet FEL pulses at the clusters, the resulting energy distribution of electrons that are knocked out from the clusters was determined using a “velocity map imaging spectrometer.”
When a material is exposed to light, electrons inside it absorb energy. In general, this energy is used to “knock electrons out” of the material. However, this can be achieved only when the energy of the light particle, i.e. “photon,” that is absorbed by the electron is greater than the energy required by the material, i.e. its “work function,” to emit the electron. Albert Einstein won a Nobel Prize in the year 1921 for explaining this “photoelectric effect.”
The research group investigated the outcome when the photon energy of the FEL light is set below the work function of clusters of neon atoms. If an electron tightly bound to a neon atom absorbs a photon of lower energy, rather than being knocked out, it becomes loosely attached, causing the atom to become “excited.”
As the FEL pulse is very intense, many electrons become loosely bound in the clusters at the same instant (many atoms become excited). As a result, the electrons are knocked out from the clusters even if the photon energy is lower than their work function.
The research group observed that a novel “cascading” process occurs when the loosely bound electrons are knocked out from the clusters.
The process is initiated when an atom possessing a loosely bound electron interacts with a neighboring atom also having a loosely bound electron. Energy is transferred from the first atom to the neighboring atom, which consequently “knocks down” its own loosely bound electron that is suspended in a “high-energy” orbit into a “low-energy” orbit located close to the atom’s core.
At the same time, the loosely bound electron in the neighboring atom is knocked out by the transferred energy. Then, the first atom, now in a “less excited” state, interacts with one more neighboring excited atom and transfers energy to it, thereby “de-exciting” itself even more and knocking an electron from the other atom. When this cascading process takes place in multiple pairs of excited atoms, a large number of low-energy electrons are emitted.
The cascades of knocking electrons out and down produce more electrons and more ions, damaging the sample more. I am convinced these cascades might play a crucial role in future radiation therapy.
Lorenz Cederbaum, Heidelberg University
The emission of low-energy electrons exposed to intense light has the ability to damage DNA, and this principle is applied in cancer radiation therapy. The outcomes of the research can have a wider impact on the prospective application of radiation therapy in the years to come.