Feb 22 2017
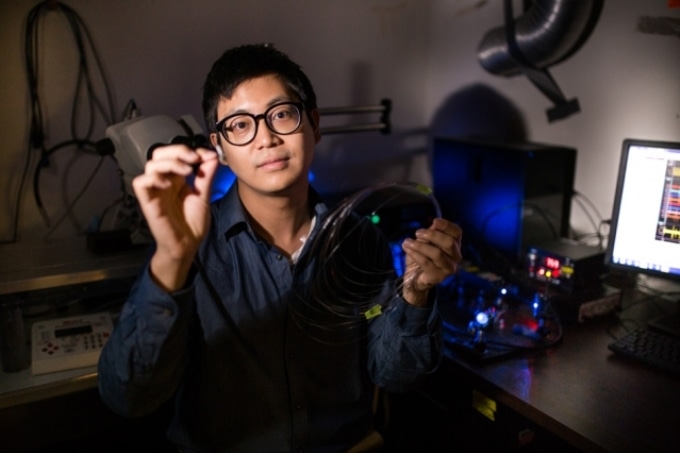 Graduate student Seongjun Park holds an example of a new flexible fiber, which is no bigger than a human hair and has successfully delivered a combination of optical, electrical, and chemical signals back and forth into the brain. (Photo credit: Young Gyu Yoon)
Graduate student Seongjun Park holds an example of a new flexible fiber, which is no bigger than a human hair and has successfully delivered a combination of optical, electrical, and chemical signals back and forth into the brain. (Photo credit: Young Gyu Yoon)
A single flexible fiber measuring no more than a human hair has, for the first time ever, effectively delivered a combination of electrical, optical, and chemical signals to and fro into the brain, implementing an idea initially suggested two years ago.
With some fine tuning to additionally enhance its biocompatibility, the new approach could provide a significantly improved means to learn about the operations and interconnections of various regions of the brain.
The new fibers were developed through a partnership among material scientists, biologists, chemists, and other experts.
The results have been published in the Nature Neuroscience journal, in a paper by Seongjun Park, an MIT graduate student; Polina Anikeeva, the Class of 1942 Career Development Professor in the Department of Materials Science and Engineering; Yoel Fink, a professor in the departments of Materials Science and Engineering, and Electrical Engineering and Computer Science; Gloria Choi, the Samuel A. Goldblith Career Development Professor in the Department of Brain and Cognitive Sciences, and 10 others at MIT and elsewhere.
The fibers are engineered to imitate the flexibility and softness of brain tissue. This could enable implants to be left in place and have them preserve their functions over longer periods than is currently possible with regular rigid, metallic fibers, enabling a lot more widespread data collection.
For instance, in lab tests on mice, the team was able to inject viral vectors that carried genes known as opsins, which sensitize neurons to light, via one of two fluid channels in the fiber.
They waited for the opsins to make an impact, then transmitted a light pulse via the optical waveguide in the center, and recorded the ensuing neuronal activity, using six electrodes to spot specific reactions. All of this was achieved through a single flexible fiber measuring only 200 μm across, which is comparable to the thickness of a human hair.
Earlier studies in neuroscience have relied on individual devices: needles for injecting viral vectors for optogenetics, optical fibers for delivering light, and arrays of electrodes for recording, adding a large amount of complication and requiring tricky alignments among the various devices. Achieving the right alignment in practice was “somewhat probabilistic,” Anikeeva says. “We said, wouldn’t it be nice if we had a device that could just do it all.”
Following years of effort, that is what the team has successfully demonstrated recently.
It can deliver the virus [containing the opsins] straight to the cell, and then stimulate the response and record the activity — and [the fiber] is sufficiently small and biocompatible so it can be kept in for a long time.
Polina Anikeeva, MIT
As each fiber is very small, “potentially, we could use many of them to observe different regions of activity,” she says. In their preliminary tests, the researchers positioned probes in two different brain regions simultaneously, changing which regions they used from experiment to experiment, and measuring the amount of time it took for responses to travel between them.
This multifunctional fiber was possible due to a main ingredient - the development of conductive “wires” that preserved the required flexibility while also transmitting electrical signals well.
The team designed a composite of conductive polyethylene doped with graphite flakes after a great deal of effort.
After a great deal work, the team could design a composite of conductive polyethylene doped with graphite flakes. The polyethylene was primarily developed into layers, sprinkled with graphite flakes, then compressed; next another pair of layers was added and compressed, and so on. Benjamin Grena, a member of the team and a recent graduate in materials science and engineering, referred to it as creating “mille feuille,” (translation - “a thousand leaves,” the French name for a Napoleon pastry).
That technique boosted the polymer’s conductivity by a factor of four or five, Park says. “That allowed us to reduce the size of the electrodes by the same amount.”
One direct question that could be answered using such fibers is: exactly how long would it take for the neurons to turn light-sensitive after injection of the genetic material? Earlier, these sorts of determinations could only be achieved using crude estimations, but presently could be pinpointed more exactly, the team explains. The specific sensitizing agent used in their primary tests was able to create effects after approximately 11 days.
The team is focused on additionally decreasing the width of the fibers, in order to make their properties a lot closer to those of the neural tissue. “The next engineering challenge is to use material that is even softer, to really match” the nearby tissue, Park says. Several research teams already want samples of the new fibers to analyze in their own research.
The research team included members of MIT’s Research Laboratory of Electronics, Department of Electrical Engineering and Computer Science, McGovern Institute for Brain Research, Department of Chemical Engineering, and Department of Mechanical Engineering, as well as researchers at Tohuku University in Japan and Virginia Polytechnic Institute.
The study was supported by the National Institute of Neurological Disorders and Stroke, the National Science Foundation, the MIT Center for Materials Science and Engineering, the Center for Sensorimotor Neural Engineering, and the McGovern Institute for Brain Research.